Abstract
Background: Actinomycetes are gram-positive bacteria that belong to the actinobacterial species. They are a prolific source of secondary metabolites with various biological applications. Thus, this study aimed to culture-based isolation of potent Actinomycete species from Sof-Umer Cave and in vitro and in vivo evaluation of their potential metabolites against selected test organisms.
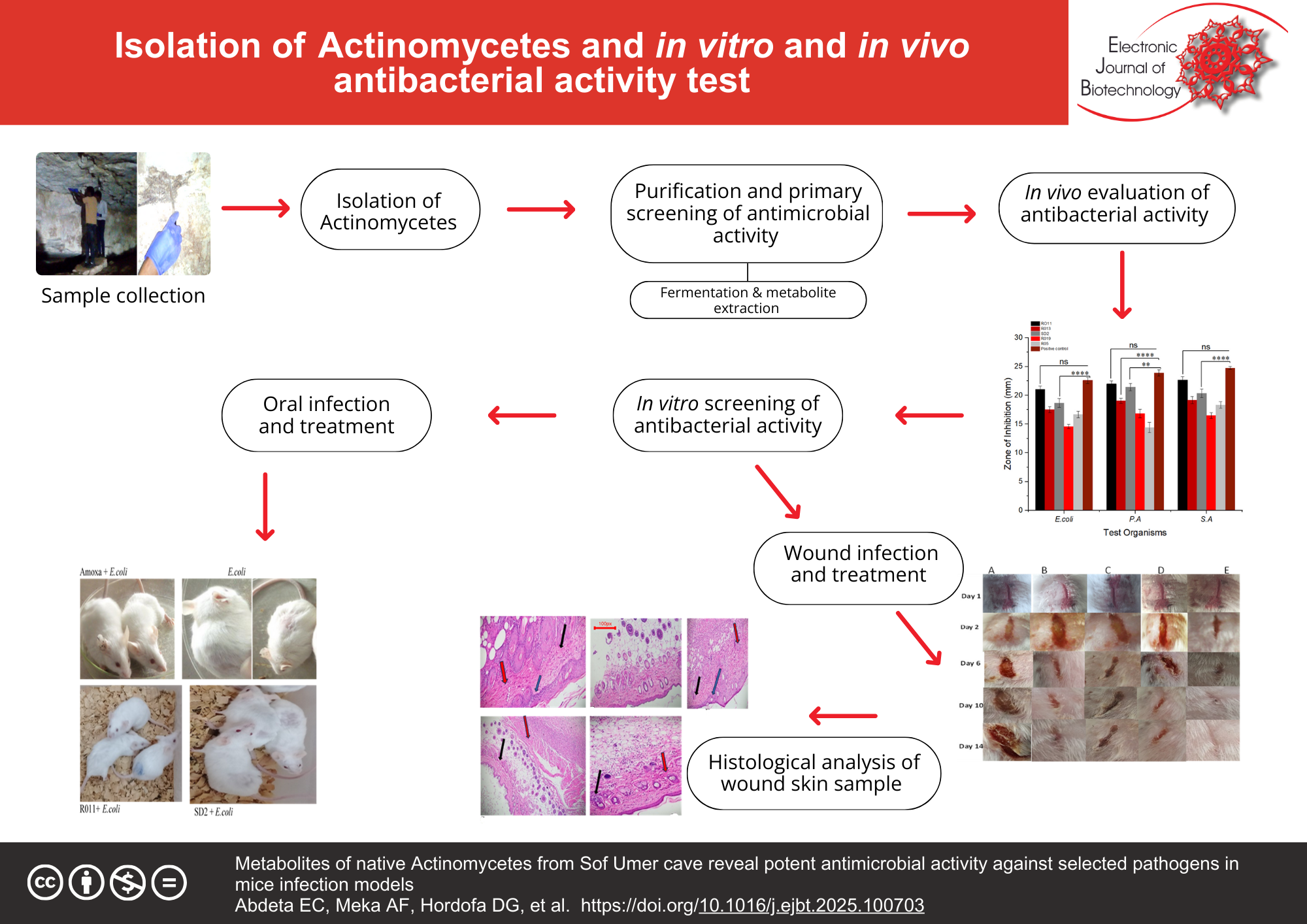
Results: Among the total isolates, ten isolates were selected based on their antimicrobial activities. Among them, the ethyl acetate crude extract of three isolates (RO13, SD2, R011) showed potential antagonistic activity, ranging from 17 ± 0.78 to 23 ± 0.56 mm of zone of inhibition against Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Additionally, two isolates’ (SD2, R011) crude extract exhibited significant inhibition of test organisms in wound and oral infection of the mice models. This was confirmed by wound contraction and progress improvement of the clinical sign observed before treatment. Characterization of their crude extract by FTIR and GC–MS revealed the presence of various functional groups and compounds. Specifically, potent antimicrobial and antioxidant bioactive compounds, such as pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-2-piperidine, phenol, 2-methoxy-4-(1-propenyl)-, and indolizine, were identified via GC–MS analysis. Three of the ten potent isolates (R013, R011, and SD2) were identified based on the 16S rRNA gene sequence, and the R013 isolate belongs to Streptomycetes flavoviridis, whereas SD2 and R011 were identified as Arthrobacter sp. and Actinobacterium kmd_152, respectively.
Conclusions: Sof-Umer cave-dwelling actinomycetes possess potent metabolites against test organisms that can be a base for future potent drug development against microbial infections.
References
. De Simeis D, Serra S. Actinomycetes: A never-ending source of bioactive compounds—An overview on antibiotics production. Antibiotics 2021;10(5):483. https://doi.org/10.3390/antibiotics10050483 PMid: 33922100
. Budhathoki S, Shrestha A. Screening of Actinomycetes from soil for antibacterial activity. Nepal J Biotechnol 2020;8(3):102–10. https://doi.org/10.3126/njb.v8i3.33664
. Ganesan P, Reegan AD, David RHA, et al. Antimicrobial activity of some actinomycetes from Western Ghats of Tamil Nadu, India. Alexandria Journal of Medicine 2017;53(2):101–10. https://doi.org/10.1016/j.ajme.2016.03.004
. Bhat MP, Nayaka S. Cave soil Streptomyces sp. strain YC69 antagonistic to chilli fungal pathogens exhibits in vitro anticancer activity against human cervical cancer cells. Appl Biochem Biotechnol 2023;195:6232–55. https://doi.org/10.1007/s12010-023-04388-y PMid: 36853440
. El-Sherebiny G, Ali ARA, Ali DM, et al. Bioengineering of zinc oxide nanoparticles as therapeutics for immunomodulatory and antimicrobial activities. Egypt J Chem 2022;65(131):1473-86. https://doi.org/10.21608/ejchem.2022.136366.6008
. Kalaba MH, El-Sherbiny GM, Ewais EA, et al. Green synthesis of zinc oxide nanoparticles (ZnO-NPs) by Streptomyces baarnensis and its active metabolite (Ka): a promising combination against multidrug-resistant ESKAPE pathogens and cytotoxicity. BMC Microbiol 2024;24:254. https://doi.org/10.1186/s12866-024-03392-4 PMid:38982372
. Busi S, Pattnaik SS. Current status and applications of actinobacteria in the production of anticancerous compounds. In: Singh BP, Gupta VK, Passari AK (editors) New and Future Developments in Microbial Biotechnology and Bioengineering 2018:137–53. https://doi.org/10.1016/B978-0-444-63994-3.00009-6.
. Nyirabahizi E, Tyson GH, Dessai U, et al. Evaluation of Escherichia coli as an indicator for antimicrobial resistance in Salmonella recovered from the same food or animal ceca samples. Food Control 2020;115:107280. https://doi.org/10.1016/j.foodcont.2020.107280
. Nawaz S, Fatima A, Saleem M, et al. Exploring the antimicrobials production potential of actinobacteria isolated from caves at Bahadurkhel Karak, Pakistan. Proceedings of the Pakistan Academy of Sciences: B Pakistan Academy of Sciences, Life and Environmental Science 2023;60(1):101-12. PMid: 35250404
. Chali K, Belay Z, Bacha K. In vitro bioassay of antibacterial and antifungal activity studies of actinomycetes from soda lakes of Ethiopia. Sys Rev Pharm 2022:13(8):798-807. https://doi.org/10.21203/rs.3.rs-668562/v1
. Elias F, Muddada S, Muleta D, et al. Antimicrobial potential of Streptomyces spp. isolated from the Rift Valley Regions of Ethiopia. Advances in Pharmacological and Pharmaceutical Sciences 2022;2022(1):1724906. https://doi.org/10.1155/2022/1724906
. Asrat A. Geology, geomorphology, geodiversity and geoconservation of the Sof Omar Cave System, Southeastern Ethiopia. Journal of African Earth Sciences 2015;108:47–63. https://doi.org/10.1016/j.jafrearsci.2015.04.015
Meka AF, Bekele GK, Abas MK, et al. Exploring microbial diversity and functional gene dynamics associated with the microbiome of Sof Umer cave, Ethiopia. Discov Appl Sci 2024;6:400. https://doi.org/10.1007/s42452-024-06110-x
. Rante H, Manggau MA, Alam G, et al. Isolation and identification of Actinomycetes with antifungal activity from karts ecosystem in Maros-Pangkep, Indonesia. Biodiversitas 2024;25(2):458-64. https://doi.org/10.13057/biodiv/d250203
. Pipite A, Lockhart PJ, McLenachan PA, et al. Isolation, antibacterial screening, and identification of bioactive cave dwelling bacteria in Fiji. Front Microbiol 2022;13:1012867. https://doi.org/10.3389/fmicb.2022.1012867.
. Almuhayawi M, Mohamed M, Abdel-Mawgoud M, et al. Bioactive potential of several actinobacteria isolated from microbiologically barely explored desert habitat, Saudi Arabia. Biology 2021;10(3):235. https://doi.org/10.3390/biology10030235
. Gatinho P, Salvador C, Gutierrez-Patricio S, et al. From cultural and natural heritage to a reservoir of biomedicine: Prospection of bioactive compounds produced by bacterial isolates from caves. International Biodeterioration & Biodegradation 2024;190:105773. https://doi.org/10.1016/j.ibiod.2024.105773
. Köksal Karayildirim Ç, ?ahiner A, Çali?kan S, et al. Isolation, identification, and antimicrobial evaluation of secondary metabolite from Serratia marcescens via an in vivo epicutaneous infection model. ACS Omega 2024;9(7):8397-404. https://doi.org/10.1021/acsomega.3c09522
. Abeje BA, Bekele T, Getahun KA, et al. Evaluation of wound healing activity of 80% hydromethanolic crude extract and solvent fractions of the leaves of Urtica simensis in mice. Journal of Experimental Pharmacology 2022;14:221–41. https://doi.org/10.2147/JEP.S363676
. Zhao X. Antimicrobial peptide MPX alleviates the lethal attack of Escherichia coli in mice. Ukrainian Journal of Veterinary and Agricultural Sciences 2021;4(3):16–21. https://doi.org/10.32718/ujvas4-3.03
. Kalaba MH, El-Sherbiny GM, Darwesh OM, et al. A statistical approach to enhance the productivity of Streptomyces baarensis MH-133 for bioactive compounds. Synthetic and Systems Biotechnology 2024;9(2):196–208. https://doi.org/10.1016/j.synbio.2024.01.012
. Rammali S, Rahim A, El Aalaoui M, et al. Antimicrobial potential of Streptomyces coeruleofuscus SCJ isolated from microbiologically unexplored garden soil in Northwest Morocco. Sci Rep 2024;14:3359. https://doi.org/10.1038/s41598-024-53801-x
. Long Y, Jiang J, Hu X, et al. Actinobacterial community in Shuanghe Cave using culture-dependent and -independent approaches. World J Microbiol Biotechnol 2019;35:153. https://doi.org/10.1007/s11274-019-2713-y
. Cheeptham N, Sadoway T, Rule D, et al. Cure from the cave: volcanic cave actinomycetes and their potential in drug discovery. Int J Speleol 2013;42(1):35–47. https://doi.org/10.5038/1827-806X.42.1.5
. Sivalingam P, Hong K, Pote J, et al. Extreme environment Streptomyces: Potential sources for new antibacterial and anticancer drug leads? International Journal of Microbiology 2019;2019(1):5283948. https://doi.org/10.1155/2019/5283948
. Zada S, Sajjad W, Rafiq M, et al. Cave microbes as a potential source of drugs development in the Modern Era. Microb Ecol 2022;84(3):676–87. https://doi.org/10.1007/s00248-021-01889-3
. Tüfekci? EF, Uzun Ü, Ertunga NS, et al. Investigation of antimicrobial activities and 16S rRNA sequences of actinomycetes isolated from karst caves in the Eastern Black Sea Region of Türkiye. Kahramanmara? Sütçü ?mam Üniversitesi Tar?m ve Do?a Dergisi 2023;26(6):1277–90. https://doi.org/10.18016/ksutarimdoga.vi.1226184
. Breijyeh Z, Jubeh B, Karaman R, et al. Resistance of Gram-Negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020;25(6):1340. https://doi.org/10.3390/molecules25061340
. Horak I, Engelbrecht G, Rensburg PJJ, et al. Microbial metabolomics: Essential definitions and the importance of cultivation conditions for utilizing Bacillus species as bionematicides. J Appl Microbiol 2019;127(2):326–43. https://doi.org/10.1111/jam.14218
. Hossain TJ. Methods for screening and evaluation of antimicrobial activity: A review of protocols, advantages, and limitations. European Journal of Microbiology and Immunology 2024;14(2):97–115. https://doi.org/10.1556/1886.2024.00035
. Nawaz S, Skala L, Amin M, et al. Genomic, molecular networking–based metabolomic, and bioactivity profiling of actinobacteria from undisturbed caves in Pakistan. Appl Biochem Biotechnol 2025;197:2667–80. https://doi.org/10.1007/s12010-024-05158-0
. Almalki FA. An overview of structure-based activity outcomes of pyran derivatives against Alzheimer’s disease. Saudi Pharmaceutical Journal 2023;31(6):998–1018. https://doi.org/10.1016/j.jsps.2023.04.030
Abeer FA-R, Mohanad JK, Imad HH, et al. Determination of bioactive chemical composition of methanolic leaves extract of Sinapis arvensis using GC-MS technique. International Journal of Toxicological and Pharmacological Research 2017;9(2):163–78
. Hillman PF, Lee C, Nam S-J. Microbial natural products with wound-healing properties. Processes 2022;11(1):30. https://doi.org/10.3390/pr11010030
. Kavitha A, Savithri HS. Biological significance of marine actinobacteria of East Coast of Andhra Pradesh, India. Front Microbiol 2017;8:1201. https://doi.org/10.3389/fmicb.2017.01201
. Shoaib M, Israyilova AA, Ganbarov K. Cyclohexane and its functionally substituted derivatives: important class of organic compounds with potential antimicrobial activities. J Microb Biotech Food Sci 2019;9(1):84–7. https://doi.org/10.15414/jmbfs.2019.9.1.84-87
. Elsayed TR, Galil DF, Sedik MZ, et al. Antimicrobial and anticancer activities of actinomycetes isolated from Egyptian soils. Int J Curr Microbiol App Sci 2020;9(9):1689–700. https://doi.org/10.20546/ijcmas.2020.909.209
. Kumari N, Menghani E, Mithal R. GC-MS analysis of compounds extracted from actinomycetes AIA6 isolates and study of its antimicrobial efficacy. Indian Journal of Chemical Technology 2019;26:362–70.
. Dehnavi F, Alizadeh SR, Ebrahimzadeh MA. Pyrrolopyrazine derivatives: Synthetic approaches and biological activities. Med Chem Res 2021;30:1981-2006. https://doi.org/10.1007/s00044-021-02792-9
. Miklasi?ska-Majdanik M, K?pa M, Wojtyczka RD, et al. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Environ. Res. Public Health 2018;15(10):2321. https://doi.org/10.3390/ijerph15102321
. Alqahtani SS, Moni SS, Sultan MH, et al. Potential bioactive secondary metabolites of Actinomycetes sp. isolated from rocky soils of the heritage village Rijal Alma, Saudi Arabia. Arabian Journal of Chemistry 2022;15:103793. https://doi.org/10.1016/j.arabjc.2022.103793
. Mothana AA, Al-Shamahy HA, Mothana RA, et al. Streptomyces sp. 1S1 isolated from Southern coast of the Red Sea as a renewable natural resource of several bioactive compounds. Saudi Pharmaceutical Journal 2022;30(2):162–71. https://doi.org/10.1016/j.jsps.2021.12.012
. Ser H-L, Palanisamy UD, Yin W-F, et al. Presence of antioxidative agent, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- in newly isolated Streptomyces mangrovisoli sp. nov. Front Microbiol 2015;6:854. https://doi.org/10.3389/fmicb.2015.00854

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Copyright (c) 2026 Electronic Journal of Biotechnology