Abstract
Background: The synthesis of keratinases by psychrophilic and psychrotolerant microorganisms has not received much attention, despite the fact that they can be an effective stand-in for substrate conversion at a low energy cost.
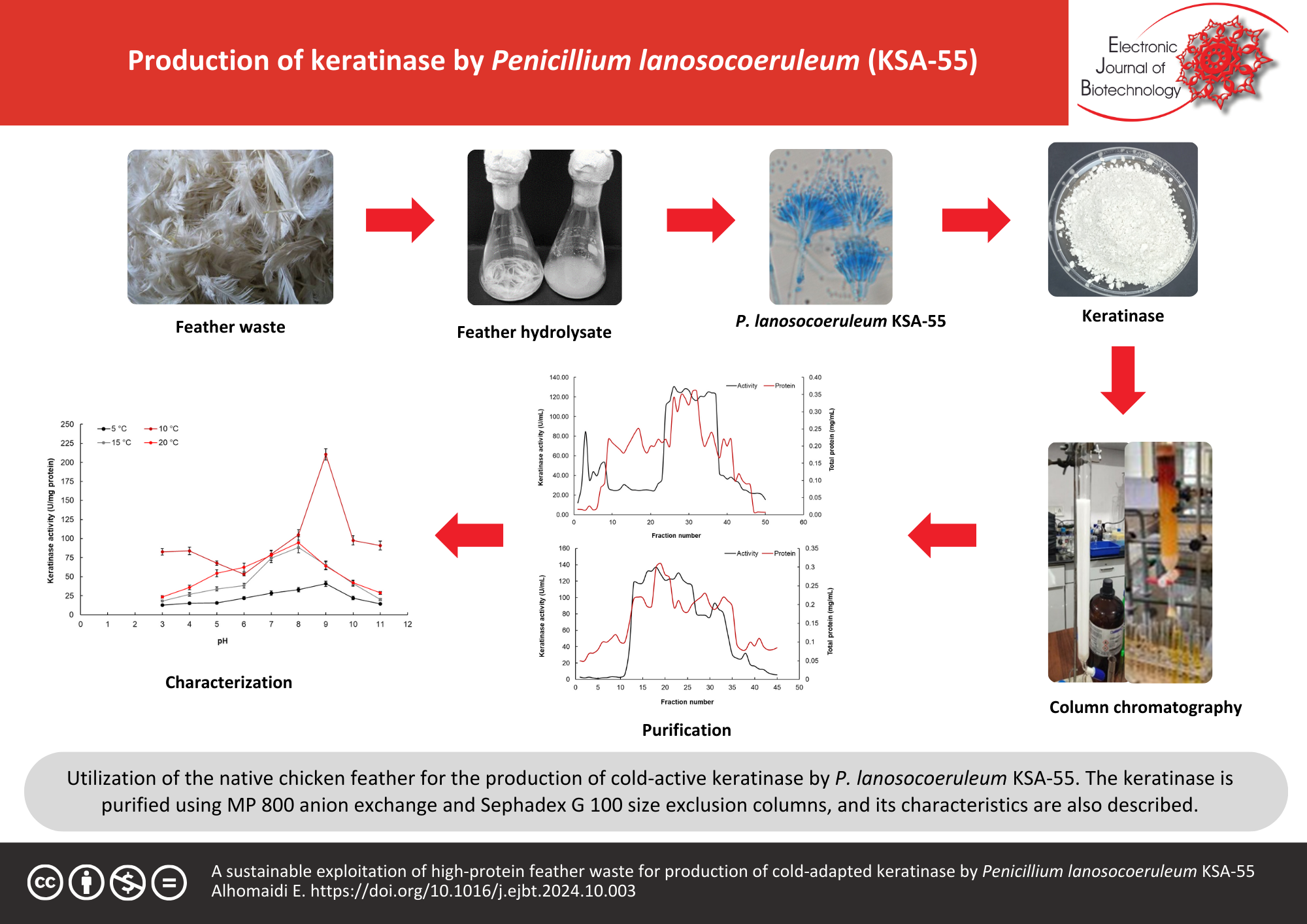
Results: In this study, some psychrophilic fungi isolated from three cold storage locations near Riyadh, Saudi Arabia, were tested for their cold-active keratinase potential. Penicillium lanosocoeruleum, a recently isolated fungus from Saudi Arabia, was the potent strain that produced cold-active keratinase. The Penicillium species was identified using sequencing of the internal transcribed spacer region (ITS). The generation of cold-active keratinase by P. lanosocoeruleum KSA-55 was optimized by two factors at time (TFAT). At pH 9.0 and 15°C, the keratinase activity was 28.9 ± 2.8 U/mL/min which increased to 41.7 ± 3.8 U/mL/min after 6 d of fermentation using peptone as a nitrogen source. The produced keratinase was chromatographed by MP 800 anion exchange resin and Sephadex G 100 size exclusion gel. At pH 9.0 and 10°C, the pure keratinase displayed the maximum specific activity of 210.3 ± 8.4 U/mg. Zn2+, Fe2+, ethylenediaminetetraacetic acid (EDTA), sodium dodecyl sulfate (SDS), and phenylmethanesulfonyl fluoride (PMSF), demonstrated severe inhibitory effects on the keratinase activity. Mn2+ ions activated the keratinase by 166.85 ± 15.6%. PMSF significantly reduced keratinase activity.
Conclusions: P. lanosocoeruleum strain KSA-55 is presented here as a new prospective producer of cold-active keratinase for a variety of biotechnological uses, including the management of keratinous waste in the poultry industry, cosmetics, and medical applications.

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Copyright (c) 2025 Electronic Journal of Biotechnology