Abstract
Background: To address public health challenges, there is increasing interest in bioactive metabolites from unconventional sources for targeted cancer and viral treatments with minimal side effects. Endophytic fungi are promising for these therapies. This study focused on maximizing bioactive agent yields from these fungi using a low-cost medium and optimized fermentation system.
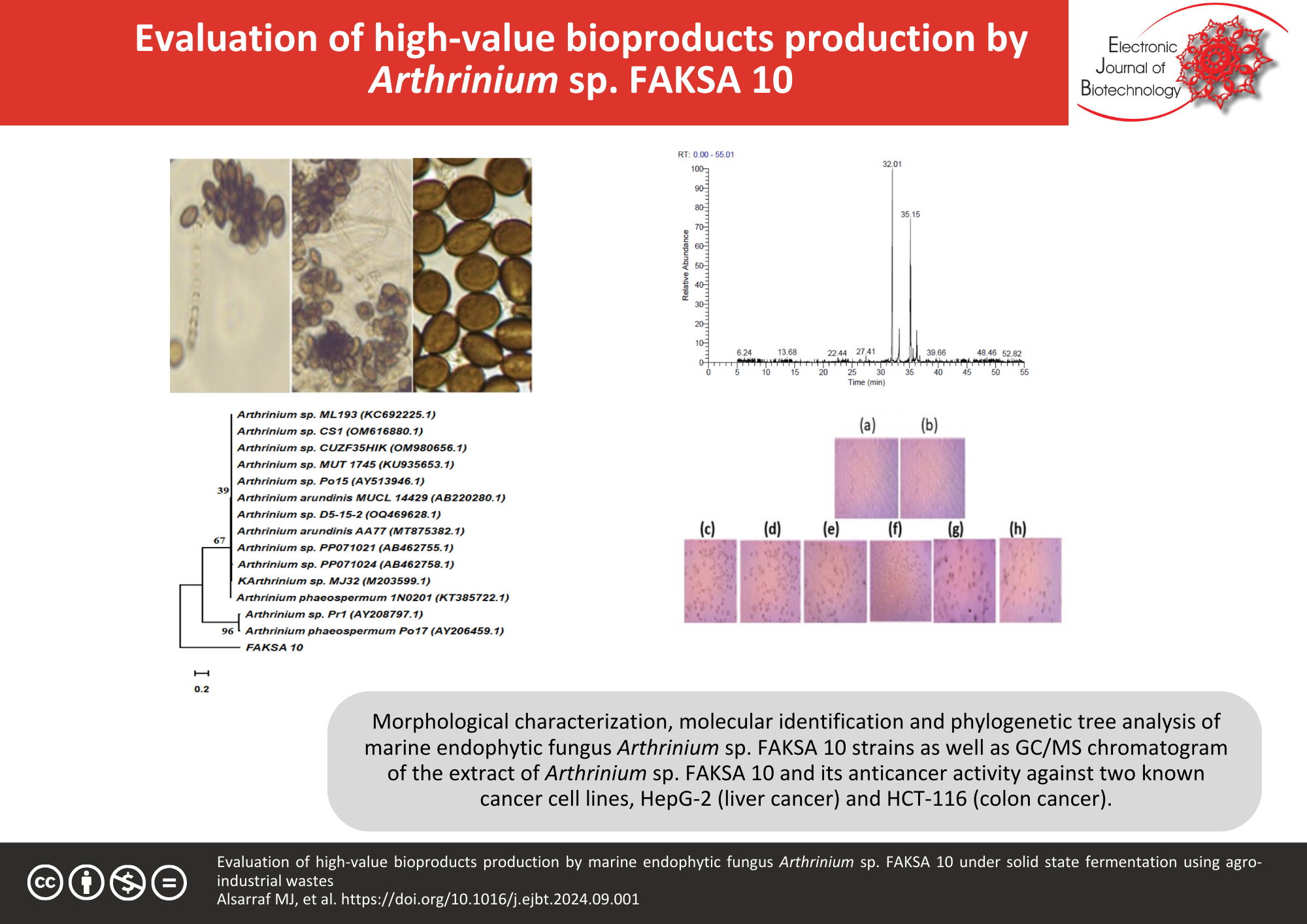
Results: Forty-three endophytic fungi isolated from Sinularia polydactyla were cultivated on wheat bran medium and evaluated for bioactive metabolites under solid-state fermentation. Strain FAKSA 10, identified as Arthrinium sp. FAKSA 10, exhibited the highest levels of pharmaceutical metabolites, including L-glutaminase, L-methioninase, L-arginase, L-asparaginase, L-tyrosinase, L-lysine α-oxidase, and ribonuclease, with a 79.12% hepatitis C virus knockdown rate. This strain produced 46 metabolites with anticancer, antioxidant, antiviral, and cytotoxic properties, including major compounds like hexadecanoic acid methyl ester; hexadecanoic acid ethyl ester; 9, 12-octadecadienoic acid (Z, Z), methyl ester; 9-octadecenoic acid (Z)-, methyl ester, and 11, 14-eicosadienoic acid, methyl ester. A cost-effective strategy using 11 agro-industrial residues was applied, followed by optimization of the solid-state fermentation system. This optimization increased enzyme yields and enhanced antiviral and antioxidant activities. The optimal conditions for solid-state fermentation were a 10 d incubation, 1 mm particle size, 60% initial moisture, 28°C temperature, and 2 × 107 CFU/mL inoculum size.
Conclusions: This study exploited Arthrinium sp. FAKSA 10 metabolites as natural pharmaceuticals against cancer and viral diseases, highlighting their significant antioxidant properties. Among various residues, oil cakes emerged as an effective and cost-efficient medium, capable of significantly enhancing the production of valuable bioactive metabolites.
References
Xu J, Y?ld?ztekin M, Han D, et al. Biosynthesis, characterization, and investigation of antimicrobial and cytotoxic activities of silver nanoparticles using Solanum tuberosum peel aqueous extract. Heliyon 2023;9(8):e19061. http://doi.org/10.1016/j.heliyon.2023.e19061
Awad MF, El-Shenawy FS, El-Gendy MMAA, et al. Purification, characterization, and anticancer and antioxidant activities of L-glutaminase from Aspergillus versicolor Faesay4. International Microbiology 2021;24(2):169-181. https://doi.org/10.1007/s10123-020-00156-8
Bender D, Koulouri A, Wen X, et al. Guanylate-binding protein 1 acts as a pro-viral factor for the life cycle of hepatitis C virus. PLoS Pathog. 2024;20(2):e1011976. https://doi.org/10.1371/journal.ppat.1011976
Alghamdi BA, Alhassan AI. The influence of Saudi board of emergency medicine residency educators on residents' academic and clinical performance in Riyadh, Saudi Arabia. Cureus. 2024;16(2):e54316. https://doi.org/10.7759/cureus.54316
El-Bondkly AAM, El-Gendy MMAA, El-Bondkly EAM, et al. Biodiversity and biological activity of fungal microbiota derived from the medicinal plants Salvia aegyptiaca L. and Balanties aegyptiaca L. Biocatalysis and Agricultural Biotechnology. 2020;28:101720. http://dx.doi.org/10.1016/j.bcab.2020.101720
Asomadu RO, Ezeorba TPC, Ezike TC, et al. Exploring the antioxidant potential of endophytic fungi: a review on methods for extraction and quantification of total antioxidant capacity (TAC). 3Biotech. 2024;14(5):127. https://doi.org/10.1007/s13205-024-03970-3
Costa M, Silva TA, Guimarães DSP. The recombinant L-lysine ?-oxidase from the fungus Trichoderma harzianum promotes apoptosis and necrosis of leukemia CD34 + hematopoietic cells. Microb Cell Fact. 2024;23:51. https://doi.org/10.1186/s12934-024-02315-2
Alzahrani NH, El-Bondkly AAM, El-Gendy MMAA, et al. Enhancement of undecylprodigiosin production from marine endophytic recombinant strain Streptomyces sp. ALAA-R20 through low-cost induction strategy. J Appl Genetics. 2021;62(1):165-182. https://doi.org/10.1007/s13353-020-00597-x
El-Gendy MMA, Awad MF, El-Shenawy FS, et al. Production, purification, characterization, antioxidant and antiproliferative activities of extracellular L-asparaginase produced by Fusarium equiseti AHMF4. Saudi J Biol Sci. 2021;28(4):2540-2548. https://doi.org/10.1016/j.sjbs.2021.01.058
Hoang PT, Luong QXT, Ayun RQ, et al. A novel approach of antiviral drugs targeting viral genomes. Microorganisms. 2022;10(8):1552. https://doi.org/10.3390/microorganisms10081552
Li J, Boix E. Host defence RNases as antiviral agents against enveloped single stranded RNA viruses. Virulence. 2021;12(1):444-469. https://doi.org/10.1080/21505594.2021.1871823
El-Gendy MMAA, Yahya SMM, Hamed AR, et al. Phylogenetic analysis and biological evaluation of marine endophytic fungi derived from Red Sea sponge Hyrtios erectus. Appl Biochem Biotechnol. 2018;185:755-777. https://doi.org/10.1007/s12010-017-2679-x
Pokrovsky VS, Chepikova OE, Davydov DZ, et al. Amino acid degrading enzymes and their application in cancer therapy. Curr Med Chem. 2019;26(3):446-464. https://doi.org/10.2174/0929867324666171006132729
Nadaf P, Kulkarni AG, Vedamurthy A. Isolation, screening and characterization of L-arginase producing soil bacteria. Int J Pharm Sci Res. 2019;10(7):3440-3444. https://doi.org/10.13040/IJPSR.0975-8232.10(7).3440-44
Lukasheva EV, Babayeva G, Karshieva SS, et al. L-lysine ?-oxidase: Enzyme with anticancer properties. Pharmaceuticals. 2021;14(11):1070. https://doi.org/10.3390/ph14111070
El-Gendy MMAA, Al-Zahrani SH, El-Bondkly, AMA. Construction of potent recombinant strain through intergeneric protoplast fusion in endophytic fungi for anticancerous enzymes production using rice straw. Appl Biochem Biotechnol. 2017;183:30-50. https://doi.org/10.1007/s12010-017-2429-0
Pintos A, Alvarado P, Planas J, et al. Six new species of Arthrinium from Europe and notes about A. caricicola and other species found in Carex spp. Hosts. Mycokeys. 2019;49:15-48. https://doi.org/10.3897/mycokeys.49.32115
Swathi AV, Dhanalakshmi SV. Optimization of process parameters for L-methioninase production in solid state fermentation by Aspergillus flavipes from sesame oil cake. Euro J Biotechnol Biosci. 2015;3:16-21.
El-Bondkly AAM, El-Gendy MMAA, El-Bondkly AMA. Construction of efficient recombinant strain through genome shuffling in marine endophytic Fusarium sp. ALAA-20 for improvement lovastatin production using agro-industrial wastes. Arabian Journal for Science and Engineering. 2021;46(1):175-190. http://dx.doi.org/10.1007/s13369-020-04925-5
Abdel-Hamid NS, Abdel-Khaleka HH, El-Shahat RM, et al. Optimization of L-asparaginase production from Fusarium oxysporum F-S3 using irradiated pomegranate peel under solid-state fermentation. Egypt J Chem. 2022;65(6):381-397. https://doi.org/10.21608/ejchem.2021.104129.4817
Javia BM, Gadhvi MS, Vyas SJ, et al. Bioprospecting of a thermostable L-methioninase from Alcaligenes aquatilis BJ-1 in agro-industrial waste. Microbiology Research. 2023;14(3):959-976. https://doi.org/10.3390/microbiolres14030066
Heo YM, Kim K, Ryu SM, et al. Diversity and ecology of marine algicolous Arthrinium species as a source of bioactive natural products. Marine Drugs. 2018;16(12):508. https://doi.org/10.3390/md16120508
El-Gendy MMA, El-Bondkly AM. Production and genetic improvement of a novel antimycotic agent, saadamycin, against dermatophytes and other clinical fungi from endophytic Streptomyces sp. Hedaya48. J Industrial Microbiol Biotechnol. 2010;37:831-841. https://doi.org/10.1007/s10295-010-0729-2 .
Dalfard AB, Khajeh K, Soudi MR, et al. Isolation and biochemical characterization of laccase and tyrosinase activities in a novel melanogenic soil bacterium. Enzyme and Microbial Technology. 2006;39(7):1409-1416. https://doi.org/10.1016/j.enzmictec.2006.03.029
Wu Y, Wang H, Ng T. Purification and characterization of a novel RNase with antiproliferative activity from the mushroom Lactarius flavidulus. J Antibiot. 2012;65:67-72. https://doi.org/10.1038/ja.2011.112
El-Gendy MMA, El-Bondkly AMA, Yahya SMM. Production and evaluation of antimycotic and antihepatitis C virus potential of fusant MERV6270 derived from mangrove endophytic fungi using novel substrates of agroindustrial wastes. Appl Biochem Biotechnol. 2014;174:2674-2701. https://doi.org/10.1007/s12010-014-1218-2.
Munsell Color. Munsell soil-color charts with genuine Munsell color chips. Munsell Color, Grand Rapids, 2009; MI, USA.
Hughes SJ. Conidiophores, conidia, and classification. Canadian Journal of Botany. 1953;31:577-659. https://doi.org/10.1139/b53-046
Minter DW. A re-appraisal of the relationships between Arthrinium and other hyphomycetes. Proc Indian Acad Sci. 1985;94:281-308. https://doi.org/10.1007/BF03053145
Cole GT. Models of cell differentiation in conidial fungi. Microbiol Rev. 1986;50:95-132. https://doi.org/10.1128/MMBR.50.2.95-132.1986
Wang M, Tan XM, Liu F, et al. Eight new Arthrinium species from China. MycoKeys. 2018;34:1-24. https://doi.org/10.3897/mycokeys.34.24221
White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds), PCR protocols: A guide to methods and applications. Academic Press, Inc., New York, 1990;315-322. http://dx.doi.org/10.1016/B978-0-12-372180-8.50042-1
El-Bondkly AMA. Molecular identification using ITS sequences and genome shuffling to improve 2-deoxyglucose tolerance and xylanase activity of marine-derived fungus, Aspergillus sp. NRCF5. Appl Biochem Biotechnol. 2012;167:2160-2173. https://doi.org/10.1007/s12010-012-9763-z
Kumar S, Stecher G, Li M, et al. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547-1549. https://doi.org/10.1093/molbev/msy096
Tamura K, Stecher G, Kumar S. MEGA 11: Molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38(7):3022-3027. https://doi.org/10.1093/molbev/msab120.
Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences 2004;101(30):11030-11035. https://doi.org/10.1073/pnas.0404206101
van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: The MTT assay. Methods Mol Biol. 2011;731:237-45. https://doi.org/10.1007/978-1-61779-080-5_20
Makris DP, Psarra E, Kallithraka S, et al. The effect of polyphenolic composition as related to antioxidant capacity in white wines. Food Res Int. 2003;36(8):805-814. https://doi.org/10.1016/S0963-9969(03)00075-9
Hue SM, Boyce AN, Somasundram C. Antioxidant activity, phenolic and flavonoid contents in the leaves of different varieties of sweet potato (Ipomoea batatas). Aust J Crop Sci. 2012;6:375-380.
Lukasheva EV, Makletsova MG, Lukashev AN, et al. Fungal enzyme L-lysine ?-oxidase affects the amino acid metabolism in the brain and decreases the polyamine level. Pharmaceuticals 2020;13(11):398. https://doi.org/10.3390/ph13110398
Lopez-Tejedor D, Claveria-Gimeno R, Velazquez-Campoy A, et al. In vitro antiviral activity of tyrosinase from mushroom Agaricus bisporus against hepatitis C virus. Pharmaceuticals. 2021;14(8):759. https://doi.org/10.3390/ph14080759
Javia BM, Gadhvi MS, Vyas SJ, et al. A review on L-methioninase in cancer therapy: Precision targeting, advancements and diverse applications for a promising future. Int J Biol Macromol. 2024;265(Pt 2):130997. https://doi.org/10.1016/j.ijbiomac.2024.130997
Vineetha MS, Aldabaan NA, More SS, et al. Production and optimization of L-glutaminase from halophilic Fusarium solani-melongenae strain CRI 24 under submerged and solid state fermentation. J Pure Appl Microbiol. 2024;18(1):593-604. https://doi.org/10.22207/JPAM.18.1.43
Li S, Yu Y, Xie P, et al. Antifungal activities of L-methionine and L-arginine treatment in vitro and in vivo against Botrytis cinerea. Microorganisms. 2024;12(2):360. https://doi.org/10.3390/microorganisms12020360
Gotte G, Menegazzi M. Biological activities of secretory RNases: Focus on their oligomerization to design antitumor drugs. Front Immunol. 2019;10:2626. https://doi.org/10.3389/fimmu.2019.02626
Burrack KS, Morrison TE. The role of myeloid cell activation and arginine metabolism in the pathogenesis of virus-induced diseases. Front Immunol. 2014;5:428. https://doi.org/10.3389/fimmu.00428
Srivastava A, Srivastava AK, Vishwkarma A, et al. Synthesis, spectroscopic studies and biological aspects of bis (cyclopentadienyl) titanium (IV) complexes with 4-amino-5-(nicotinic/picolinic/isonicotinic/indole-3-propyl/indole-3-ethyl)-3-mercapto-1,2,4-triazole. J Indian Chem Soc. 2020;97(11b):2363-2371.
El-Sayed ER, Hazaa MA, Shebl MM, et al. Bioprospecting endophytic fungi for bioactive metabolites and use of irradiation to improve their bioactivities. AMB Express. 2022;12(1):46. https://doi.org/10.1186/s13568-022-01386-x
Dandash F, Leger DY, Diab-Assaf M, et al. Porphyrin/chlorin derivatives as promising molecules for therapy of colorectal cancer. Molecules. 2021;26(23):7268. https://doi.org/10.3390/molecules26237268
Morland C, Nordengen K. N-Acetyl-aspartyl-glutamate in brain health and disease. Int J Mol Sci. 2022;23(3):1268. https://doi.org/10.3390/ijms23031268
Stradiotto A, Kosoko-Lasaki S. Glaucoma pharmacology. In Handbook of Basic and Clinical Ocular Pharmacology and Therapeutics, Sunny EO, Najam AS, 2022; Academic Press, Elsevier Inc. ISBN978-0-12-819291-7. https://doi.org/10.1016/C2018-0-05318-9
Crous PW, Groenewald JZ. A phylogenetic re-evaluation of Arthrinium. IMA Fungus. 2013;4:133-154. https://doi.org/10.5598/imafungus.2013.04.01.13
Elissawy AM, Ebada SS, Ashour ML, et al. Spiroarthrinols A and B, two novel meroterpenoids isolated from the sponge-derived fungus Arthrinium sp. Phytochem Lett. 2017;20:246-251. https://doi.org/10.1016/j.phytol.2017.05.008
Shu Y, Wang JP, Li BX, et al. Bioactive cytochalasans from the fungus Arthrinium arundinis DJ-13. Phytochemistry. 2022;194:113009. https://doi.org/10.1016/j.phytochem.2021.113009
Ebada SS, Schulz B, Wray V, et al. Arthrinins A-D: Novel diterpenoids and further constituents from the sponge derived fungus Arthrinium sp. Bioorg Med Chem. 2011;19(15):4644-4651. https://doi.org/10.1016/j.bmc.2011.06.013
Hong JH, Jang S, Heo YM, et al. Investigation of marine-derived fungal diversity and their exploitable biological activities. Mar Drugs. 2015;13:4137-4155. https://doi.org/10.3390/md13074137
Bao J, He F, Yu JH, et al. New chromones from a marine-derived fungus, Arthrinium sp., and their biological activity. Molecules. 2018;23(8):1982. https://doi.org/10.3390/molecules23081982

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Copyright (c) 2025 Electronic Journal of Biotechnology