Abstract
Background: Eco-friendly synthesis of silver nanoparticles (AgNPs) using biological systems offers a sustainable alternative to conventional physicochemical methods. In this study, we employed cell-free extracts from three thermotolerant bacterial strains, Bacillus haynesii CamB6, Pseudomonas alcaligenes Med1, and Staphylococcus sp. BSP3 for the biosynthesis of AgNPs, aiming to explore their antioxidant and antibacterial properties.
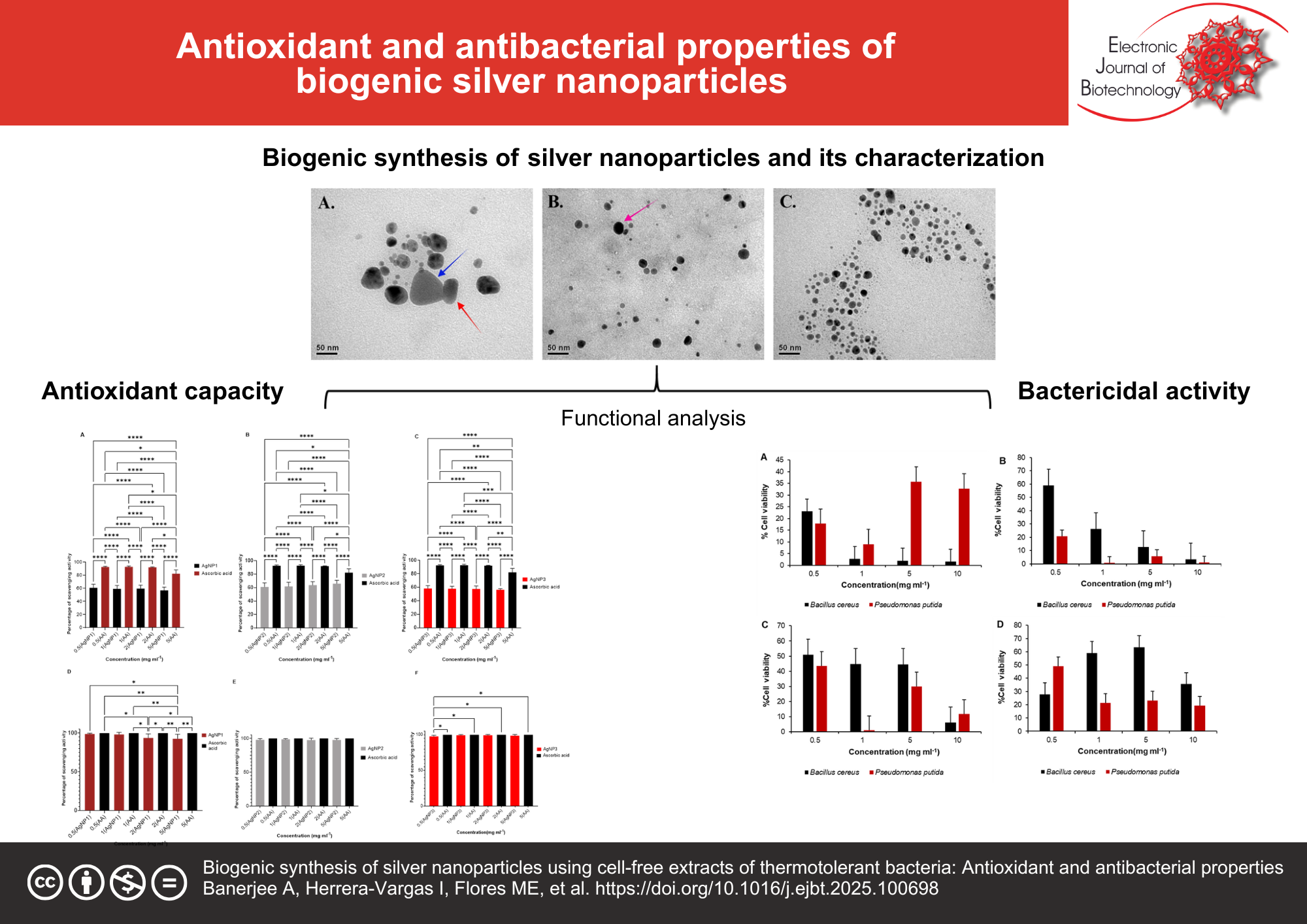
Results: The biosynthesized AgNPs were characterized through UV–Vis spectroscopy, FTIR, TEM, and DLS analyses, which revealed distinct physicochemical profiles among the nanoparticles. Notably, AgNP2 and AgNP3 exhibited smaller particle sizes, enhanced colloidal stability, and superior biological activities compared to AgNP1. Antioxidant evaluation demonstrated significant free radical scavenging potential, with AgNP2 showing the highest DPPH activity (65.18% at 5 mg mL−1). Antibacterial activity, assessed via agar well diffusion and cell viability assays against Bacillus cereus and Pseudomonas putida revealed that AgNP2 achieved the lowest bacterial viability (0.74%) for P. putida at 1 mg mL−1 concentration.
Conclusions: The study highlights the potential of biosynthesized AgNPs, particularly AgNP2, as sustainable for biomedical applications. Their antioxidant and antibacterial activities suggest valuable applications in managing oxidative stress and combating antimicrobial resistance.
References
Malik, S., Muhammad, K., Waheed, Y. Emerging applications of nanotechnology in healthcare and medicine. Molecules 2023;28(18):6624. https://doi.org/10.3390/molecules28186624 PMid: 37764400
Mandvi, Singh PP, Ballal S, et al. Construction of a 3D flower-like NiO/Mn?O? heterojunction using Tulsi leaf extract for enhanced photodegradation of thiamethoxam pesticide and organic dyes under direct sunlight. Materials Advances 2024;5(20):8097–8110. https://doi.org/10.1039/D4MA00708E
Sodhi RS, Singh PP, Lal B, et al. Biogenic synthesis of ZnO nanoparticles using Polystichum squarrosum extract and its applications as anti-oxidant, anti-diabetic agent and industrial wastewater treatment. Emergent Materials 2024;7:285–298. https://doi.org/10.1007/s42247-023-00589-7
Kumari, V., Kaushal, S., Singh, PP. Green synthesis of a CuO/rGO nanocomposite using a Terminalia arjuna bark extract and its catalytic activity for the purification of water. Materials Advances 2022;3(4):2170–2184. https://doi.org/10.1039/D1MA00993A
Mahajan M., Kumar S, Gaur J, et al. Role of cellulose, phenolic compounds, and water-soluble proteins in ZnO nanoparticle synthesis using Mangifera indica leaf extract for photocatalytic and antioxidant investigations. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2025;720:137066. https://doi.org/10.1016/j.colsurfa.2025.137066
Mahajan, M., Kumar, R., Gaur J, et al. Green synthesis of ZnO nanoparticles using Justicia adhatoda for photocatalytic degradation of malachite green and reduction of 4-nitrophenol. RSC Advances 2025;15(4):2958–2980. https://doi.org/10.1039/D4RA08632E
Kumar, V., Singh, Y., Kaushal, S., et al. Bioinspired synthesis of copper oxide nanoparticles using aqueous extracts of Cladophora glomerata (L.) Kuetz and their potential biomedical applications. Bioprocess and Biosystems Engineering 2025;48:633–646. https://doi.org/10.1007/s00449-025-03133-5 PMid: 39928099
Singh, H., Du, J., Singh, P., et al. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artificial Cells, Nanomedicine, and Biotechnology 2018;46(6):1163–1170. https://doi.org/10.1080/21691401.2017.1362417 PMid: 28784039
Ahmed S, Annu, Ikram S, et al. Biosynthesis of gold nanoparticles: A green approach. Journal of Photochemistry and Photobiology B: Biology, 2016;161:141–153. https://doi.org/10.1016/j.jphotobiol.2016.04.034 PMid: 27236049
Murugan, S., Senthilvelan, T., Govindasamy, M., et al. A comprehensive review on exploring the potential of phytochemicals and biogenic nanoparticles for the treatment of antimicrobial-resistant pathogenic bacteria. Current Microbiology, 2025;82(2):90. https://doi.org/10.1007/s00284-025-04064-w PMid:39825917
Gurunathan, S., Han, J.W., Kwon, D.-N., et al. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Research Letters 2014;9(1):373. https://doi.org/10.1186/1556-276X-9-373 PMid: 25136281
Banerjee A, Das D, Andler R, et al. Green synthesis of silver nanoparticles using exopolysaccharides produced by Bacillus anthracis PFAB2 and its biocidal property. Journal of Polymers and the Environment, 2021;29(8):2701–2709. https://doi.org/10.1007/s10924-021-02051-3
Ravindran, A., Chandran, P., Khan, S.S. Biofunctionalized silver nanoparticles: Advances and prospects. Colloids and Surfaces B: Biointerfaces, 2013;105:342–352. https://doi.org/10.1016/j.colsurfb.2012.07.036 PMid:2 3411404
Dheyab MA., Aziz AA, Jameel MS, et al. Sustainable green synthesis of silver nanoparticles for safer biomedical application. Journal of Environmental Chemical Engineering, 2025;13(2):115998. https://doi.org/10.1016/j.jece.2025.115998
Taran M, Rad M, Alavi M. Antibacterial activity of copper oxide (CuO) nanoparticles biosynthesized by Bacillus sp. FU4: Optimization of experiment design. Pharmaceutical Sciences, 2014;23(3):198–206. https://doi.org/10.15171/PS.2017.30
Das CGA, Sharma R, Singh A, et al. Antibacterial activity of silver nanoparticles (biosynthesis): A short review on recent advances. Biocatalysis and Agricultural Biotechnology, 2020;27:101593. https://doi.org/10.1016/j.bcab.2020.101593
Galdiero, S., Falanga, A., Vitiello, M., et al. Silver nanoparticles as potential antiviral agents. Molecules, 2011;16(10):8894–8918. https://doi.org/10.3390/molecules16108894 PMid: 22024958
Pangli, H., Vatanpour, S., Hortamani, S., et al. Incorporation of silver nanoparticles in hydrogel matrices for controlling wound infection. Journal of Burn Care & Research, 2021;42(4):785–793. https://doi.org/10.1093/jbcr/iraa205 PMid: 33313805
Gondil VS, Kalaiyarasan T, Bharti VK, et al. Antibiofilm potential of Seabuckthorn silver nanoparticles (SBT@AgNPs) against Pseudomonas aeruginosa. 3 Biotech, 2019;9(11):402. https://doi.org/10.1007/s13205-019-1947-6 PMid: 31681523
Khairnar SV, Das A, Oupický D, et al. Strategies to overcome antibiotic resistance: Silver nanoparticles and vancomycin in pathogen eradication. RSC Pharmaceutics, 2025;2(3):455–479. https://doi.org/10.1039/D4PM00314D
Singh, Y., Kaushal, S., Sodhi, R. S. Biogenic synthesis of silver nanoparticles using cyanobacterium Leptolyngbya sp. WUC 59 cell-free extract and their effects on bacterial growth and seed germination. Nanoscale Advances, 2020;2(9):3972–3982. https://doi.org/10.1039/D0NA00357C PMid: 36132754
Reidy B, Haase A, Luch A, et al. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials, 2013;6(6):2295–2350. https://doi.org/10.3390/ma6062295 PMid: 28809275
Casals E, Gusta MF, Bastus N, et al. Silver nanoparticles and antibiotics: A promising synergistic approach to multidrug-resistant infections. Microorganisms, 2025;13(4):952. https://doi.org/10.3390/microorganisms13040952 PMid: 40284788
Yamanaka M, Hara K, Kudo J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Applied and Environmental Microbiology, 2005;71(11):7589–7593. https://doi.org/10.1128/AEM.71.11.7589-7593.2005 PMid: 16269810
Banerjee A, Roy RK, Sarkar S, et al. Synthesis of hot spring origin bacterial cell wall polysaccharide-based copper nanoparticles with antibacterial property. Electronic Journal of Biotechnology, 2024;68:11–19. https://doi.org/10.1016/j.ejbt.2023.11.005
Banerjee, A., Das, D., Rudra, S. G., et al. Characterization of exopolysaccharide produced by Pseudomonas sp. PFAB4 for synthesis of EPS-coated AgNPs with antimicrobial properties. Journal of Polymers and the Environment, 2020;28(1):242–256. https://doi.org/10.1007/s10924-019-01602-z
Banerjee A, Mohammed Breig SJ, Gómez A, et al. Optimization and characterization of a novel exopolysaccharide from Bacillus haynesii CamB6 for food applications. Biomolecules, 2022;12(6):834. https://doi.org/10.3390/biom12060834 PMid: 35740959
Sarkar S, Cabrera-Barjas G, Singh RN, et al. Unveiling a novel exopolysaccharide produced by Pseudomonas alcaligenes Med1 isolated from a Chilean hot spring as biotechnological additive. Scientific Reports, 2024;14(1):25058. https://doi.org/10.1038/s41598-024-74830-6 PMid: 39443539
Banerjee, S., Cabrera-Barjas, G., Tapia, J., et al. Characterization of Chilean hot spring-origin Staphylococcus sp. BSP3 produced exopolysaccharide as biological additive. Natural Products and Bioprospecting 2024;14(1):15. https://doi.org/10.1007/s13659-024-00436-0 PMid: 38310179
Ghodake G, Kim M, Sung J, et al. Extracellular synthesis and characterization of silver nanoparticles—Antibacterial activity against multidrug-resistant bacterial strains. Nanomaterials 2020;10(2):360. https://doi.org/10.3390/nano10020360 PMid: 32092941
Singh, P., Mijakovic, I. Strong antimicrobial activity of silver nanoparticles obtained by the green synthesis in Viridibacillus sp. extracts. Frontiers in Microbiology, 2022;13:820048. https://doi.org/10.3389/fmicb.2022.820048 PMid: 35250934
Nas F, Aissaoui N, Mahjoubi M, et al. A comparative GC–MS analysis of bioactive secondary metabolites produced by halotolerant Bacillus spp. isolated from the Great Sebkha of Oran. International Microbiology 2021;24(3):455–470. https://doi.org/10.1007/s10123-021-00185-x PMid: 34100180
Jacob JA, Mahal HS, Biswas N, et al. Role of phenol derivatives in the formation of silver nanoparticles. Langmuir 2008;24(2):528–533. https://doi.org/10.1021/la702073r PMid: 18095719
Ansari MA, Asiri SMM, Alzohairy MA, et al. Biofabricated fatty acids-capped silver nanoparticles as potential antibacterial, antifungal, antibiofilm and anticancer agents. Pharmaceuticals, 2021;14(2):139. https://doi.org/10.3390/ph14020139 PMid: 33572296
Ashrafi-Saiedlou S, Rasouli-Sadaghiani M, Fattahi M, et al. Biosynthesis and characterization of iron oxide nanoparticles fabricated using cell-free supernatant of Pseudomonas fluorescens for antibacterial, antifungal, antioxidant, and photocatalytic applications. Scientific Reports 2025;15(1):1018. https://doi.org/10.1038/s41598-024-84974-0 PMid: 39762412
Fedorova, M. S., Mironova, A. V., Kayumov, A. R., et al. Cell-free supernatant of Staphylococcus aureus culture increases antimicrobials susceptibility of Pseudomonas aeruginosa. Opera Medica et Physiologica, 2022;9(3):113–120. https://doi.org/10.24412/2500-2295-2022-3-113-120
Siriwardana K, Wang A, Gadogbe M, et al. Studying the effects of cysteine residues on protein interactions with silver nanoparticles. The Journal of Physical Chemistry C, 2015;119(5):2910–2916. https://doi.org/10.1021/jp512440z PMid: 26207157
Atalah, J., Espina, G., Blamey, L., et al. Advantages of using extremophilic bacteria for the biosynthesis of metallic nanoparticles and its potential for rare earth element recovery. Frontiers in Microbiology, 2022;13:855077. https://doi.org/10.3389/fmicb.2022.855077 PMid: 35387087
Focardi S, Pepi M, Landi G, et al. Hexavalent chromium reduction by whole cells and cell-free extract of the moderate halophilic bacterial strain Halomonas sp. TA-04. International Biodeterioration & Biodegradation, 2012;66(1):63–70. https://doi.org/10.1016/j.ibiod.2011.11.003
Shimada, K., Fujikawa, K., Yahara, K., et al. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. Journal of Agricultural and Food Chemistry, 1992;40(6):945–948. https://doi.org/10.1021/jf00018a005
Ruch, R. J., Cheng, S., Klaunig, J. E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis, 1986;10(6):1003–1008. https://doi.org/10.1093/carcin/10.6.1003 PMid: 2470525
Chavez-Esquivel, G., Cervantes-Cuevas, H., Ybieta-Olvera, L. F., et al. Antimicrobial activity of graphite oxide doped with silver against Bacillus subtilis, Candida albicans, Escherichia coli, and Staphylococcus aureus by agar well diffusion test: Synthesis and characterization. Materials Science and Engineering: C, 2021;123:111934. https://doi.org/10.1016/j.msec.2021.111934 PMid: 33812573
Gallagher, S.R. Digital image processing and analysis with ImageJ. Current Protocols Essential Laboratory Techniques, 2014;9(1):A.3C.1-A.3C.29. https://doi.org/10.1002/9780470089941.eta03cs9
Saha, N., Trivedi, P., Dutta Gupta, S. Surface plasmon resonance (SPR) based optimization of biosynthesis of silver nanoparticles from rhizome extract of Curculigo orchioides Gaertn. and its antioxidant potential. Journal of Cluster Science, 2016;27(6):1893–1912. https://doi.org/10.1007/s10876-016-1050-7
Kaimuangpak, K., Tamprasit, K., Date, A., et al. Synthesis of bioactive spherical silver nanoparticles with surface plasmon resonance using ethanolic twig extract of Cratoxylum formosum ssp. pruniflorum. Journal of Drug Delivery Science and Technology, 2023;88:104897. https://doi.org/10.1016/j.jddst.2023.104897
Gusrizal, G., Santosa, S. J., Kunarti, E. S., et al. Synthesis of silver nanoparticles by reduction of silver ion with m-hydroxybenzoic acid. Asian Journal of Chemistry, 2017;29(7):1417–1422. https://doi.org/10.14233/ajchem.2017.20436
Marguier A, Lakard S, Soraru C, et al. Modulation by surroundings of the antibacterial efficiency of silver in water environments. Journal of Nanoparticle Research 2019;21(6):129. https://doi.org/10.1007/s11051-019-4544-z
Choi, Y., Kim, H.-A., Kim, K.-W., et al. Comparative toxicity of silver nanoparticles and silver ions to Escherichia coli. Journal of Environmental Sciences, 2018;66:50–60. https://doi.org/10.1016/j.jes.2017.04.028 PMid: 29628108
Chen, Y., Chen, Y., Liu, L., et al. Microbial synthesis of 4-hydroxybenzoic acid from renewable feedstocks. Food Chemistry: Molecular Sciences, 2021;3:100059. https://doi.org/10.1016/j.fochms.2021.100059 PMid: 35415641
Wang, K., Pan, X., Yang, T., et al. Efficient production of salicylic acid through CmeR-PcmeO biosensor-assisted multiplexing pathway optimization in Escherichia coli. Biotechnology for Biofuels and Bioproducts, 2025;18(1):40. https://doi.org/10.1186/s13068-025-02637-2 PMid: 40156043
Karthik, L., Kumar, G., Kirthi, A. V., et al. Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioprocess and Biosystems Engineering, 2014;37(2):261–267. https://doi.org/10.1007/s00449-013-0994-3 PMid: 23771163
Sidhu, A. K., Verma, N., Kaushal, P. Role of biogenic capping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Frontiers in Nanotechnology, 2022;3:801620. https://doi.org/10.3389/fnano.2021.801620
Saeed S, Iqbal A, Ashraf MA. Bacterial-mediated synthesis of silver nanoparticles and their significant effect against pathogens. Environmental Science and Pollution Research, 2020;27(30):37347–37356. https://doi.org/10.1007/s11356-020-07610-0 PMid: 32130634
Jia Z, Li J, Gao L, et al. Dynamic light scattering: A powerful tool for in situ nanoparticle sizing. Colloids and Interfaces 2023;7(1):15. https://doi.org/10.3390/colloids7010015
Rodriguez-Loya J, Lerma M, Gardea-Torresdey JL. Dynamic light scattering and its application to control nanoparticle aggregation in colloidal systems: A review. Micromachines, 2023;15(1):24. https://doi.org/10.3390/mi15010024 PMid: 38258143
Filippov, S.K., R Khusnutdinov, A. Murmiluk et al. Dynamic light scattering and transmission electron microscopy in drug delivery: A roadmap for correct characterization of nanoparticles and interpretation of results. Materials Horizons, 2023;10(12):5354–5370. https://doi.org/10.1039/D3MH00717K PMid: 37814922
Danaei, M., M. Dehghankhold, S Ataei, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics, 2018;10(2):57. https://doi.org/10.3390/pharmaceutics10020057 PMid: 29783687
Wang, Z., Xiao, K., Wang, X. Role of coexistence of negative and positive membrane surface charges in electrostatic effect for salt rejection by nanofiltration. Desalination, 2018;444:75–83. https://doi.org/10.1016/j.desal.2018.07.010
Sadowski, Z., Maliszewska, I. H., Grochowalska, et al. Synthesis of silver nanoparticles using microorganisms. Materials Science-Poland, 2008;26(2):419-424.
Fouad, H, L. Honjie, D. Yanmei et al. Synthesis and characterization of silver nanoparticles using Bacillus amyloliquefaciens and Bacillus subtilis to control filarial vector Culex pipiens pallens and its antimicrobial activity. Artificial Cells, Nanomedicine, and Biotechnology, 2017;45(7):1369–1378. https://doi.org/10.1080/21691401.2016.1241793 PMid: 27855517
Ansari, A., S. Pervez, U. Javed et al. Characterization and interplay of bacteriocin and exopolysaccharide-mediated silver nanoparticles as an antibacterial agent. International Journal of Biological Macromolecules, 2018;115:643–650. https://doi.org/10.1016/j.ijbiomac.2018.04.104 PMid: 29689285
Halawani, E. M., Hassan, A. M., Gad El-Rab, S. M. Nanoformulation of biogenic cefotaxime-conjugated-silver nanoparticles for enhanced antibacterial efficacy against multidrug-resistant bacteria and anticancer studies. International Journal of Nanomedicine, 2020;15:1889–1901. https://doi.org/10.2147/IJN.S236182 PMid: 32256066
Javaid, S., N. M Ahmad, A Mahmood et al. Cefotaxime loaded polycaprolactone based polymeric nanoparticles with antifouling properties for in-vitro drug release applications. Polymers, 2021;13(13):2180. https://doi.org/10.3390/polym13132180 PMid: 34209144
Fontana R, Ligozzi M, Comaglia G. Affinities of cephalosporins for penicillin-binding proteins and their antibacterial activities in the presence of human serum albumin. Clin Microbiol Infect, 2000;6(3):82-83. https://doi.org/10.1111/j.1469-0691.2000.tb02052.x
Babayevska, N., ?. Przysiecka, I Iatsunskyi et al. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Scientific Reports, 2022;12(1):8148. https://doi.org/10.1038/s41598-022-12134-3 PMid: 35581357
Mohammed, A. M., Hassan, K. T., Hassan, O. M. Assessment of antimicrobial activity of chitosan/silver nanoparticles hydrogel and cryogel microspheres. International Journal of Biological Macromolecules, 2023;233:123580. https://doi.org/10.1016/j.ijbiomac.2023.123580 PMid: 36764343
Wangoye, K., Mwesigye, J., Tungotyo, M., et al. Chronic wound isolates and their minimum inhibitory concentrations against third generation cephalosporins at a tertiary hospital in Uganda. Scientific Reports, 2022;12(1):1195. https://doi.org/10.1038/s41598-021-04722-6 PMid: 35075152
Singh, R., Shedbalkar, U. U., Wadhwani, S. A., et al. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Applied Microbiology and Biotechnology, 2015;99(11):4579–4593. https://doi.org/10.1007/s00253-015-6622-1 PMid: 25952110
Parmar, S., Kaur, H., Singh, J., et al. Recent advances in green synthesis of Ag NPs for extenuating antimicrobial resistance. Nanomaterials, 2022;12(7):1115. https://doi.org/10.3390/nano12071115 PMid: 35407234
Yalcinoz, A. H., Gursoy Calis, G. E., Karaca, B., et al. Biosynthesis of silver nanoparticles by thermophilic spore-forming bacilli: Screening for high-performance strains and characterization of silver nanoparticles from Anoxybacillus sp. D401a. Preparative Biochemistry & Biotechnology, 2025;1–23. https://doi.org/10.1080/10826068.2025.2532470 PMid: 40680066
Srimathi, R., Sondak, T., Kim, K. Cell-free supernatant-assisted biogenic silver nanoparticles enhance the antibacterial efficacy of communicating bacterial pathogens. Biotechnology and Bioprocess Engineering, 2024;29(5):902–914. https://doi.org/10.1007/s12257-024-00122-5
Savvidou, M. G., Kontari, E., Kalantzi, S., et al. Green synthesis of silver nanoparticles using the cell-free supernatant of Haematococcus pluvialis culture. Materials, 2023;17(1):187. https://doi.org/10.3390/ma17010187 PMid: 38204044
Dolai, J., Mandal, K., Jana, N. R. Nanoparticle size effects in biomedical applications. ACS Applied Nano Materials, 2021;4(7):6471–6496. https://doi.org/10.1021/acsanm.1c00987
Masarudin, M. J., Cutts, S. M., Evison, B. J., et al. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [14C]-doxorubicin. Nanotechnology, Science and Applications, 2015;8:67-80. https://doi.org/10.2147/NSA.S91785 PMid: 26715842
Haripriyaa, M., Suthindhiran, K. Pharmacokinetics of nanoparticles: Current knowledge, future directions and its implications in drug delivery. Future Journal of Pharmaceutical Sciences, 2023;9(1):113. https://doi.org/10.1186/s43094-023-00569-y
Loo, Y. Y., Y Rukayadi, M Nor-Khaizura et al. In vitro antimicrobial activity of green synthesized silver nanoparticles against selected Gram-negative foodborne pathogens. Frontiers in Microbiology, 2018;9:1555. https://doi.org/10.3389/fmicb.2018.01555 PMid: 30061871
Bogdanova, L. R., Valiullina, Y. A., Faizullin, D. A., et al. Spectroscopic, zeta potential and molecular dynamics studies of the interaction of antimicrobial peptides with model bacterial membrane. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2020;242:118785. https://doi.org/10.1016/j.saa.2020.118785 PMid: 32801024
Menichetti, A., Mavridi-Printezi, A., Mordini, D., et al. Effect of size, shape and surface functionalization on the antibacterial activity of silver nanoparticles. Journal of Functional Biomaterials, 2023;14(5):244. https://doi.org/10.3390/jfb14050244 PMid: 37233354
Modi, S. K., Gaur, S., Sengupta, M., et al. Mechanistic insights into nanoparticle surface–bacterial membrane interactions in overcoming antibiotic resistance. Frontiers in Microbiology, 2023;14:1135579. https://doi.org/10.3389/fmicb.2023.1135579 PMid: 37152753

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Copyright (c) 2026 Electronic Journal of Biotechnology