Abstract
Background: Doxorubicin (DOX), a widely used chemotherapeutic agent, causes severe cardiotoxicity that frequently progresses to dilated cardiomyopathy (DCM). While ankyrin repeat domain 1 protein (ANKRD1) plays critical roles in cardiovascular pathophysiology, its specific involvement in doxorubicin-induced DCM remains unknown. This study investigates the functional significance of ANKRD1 in DOX-induced DCM pathogenesis.
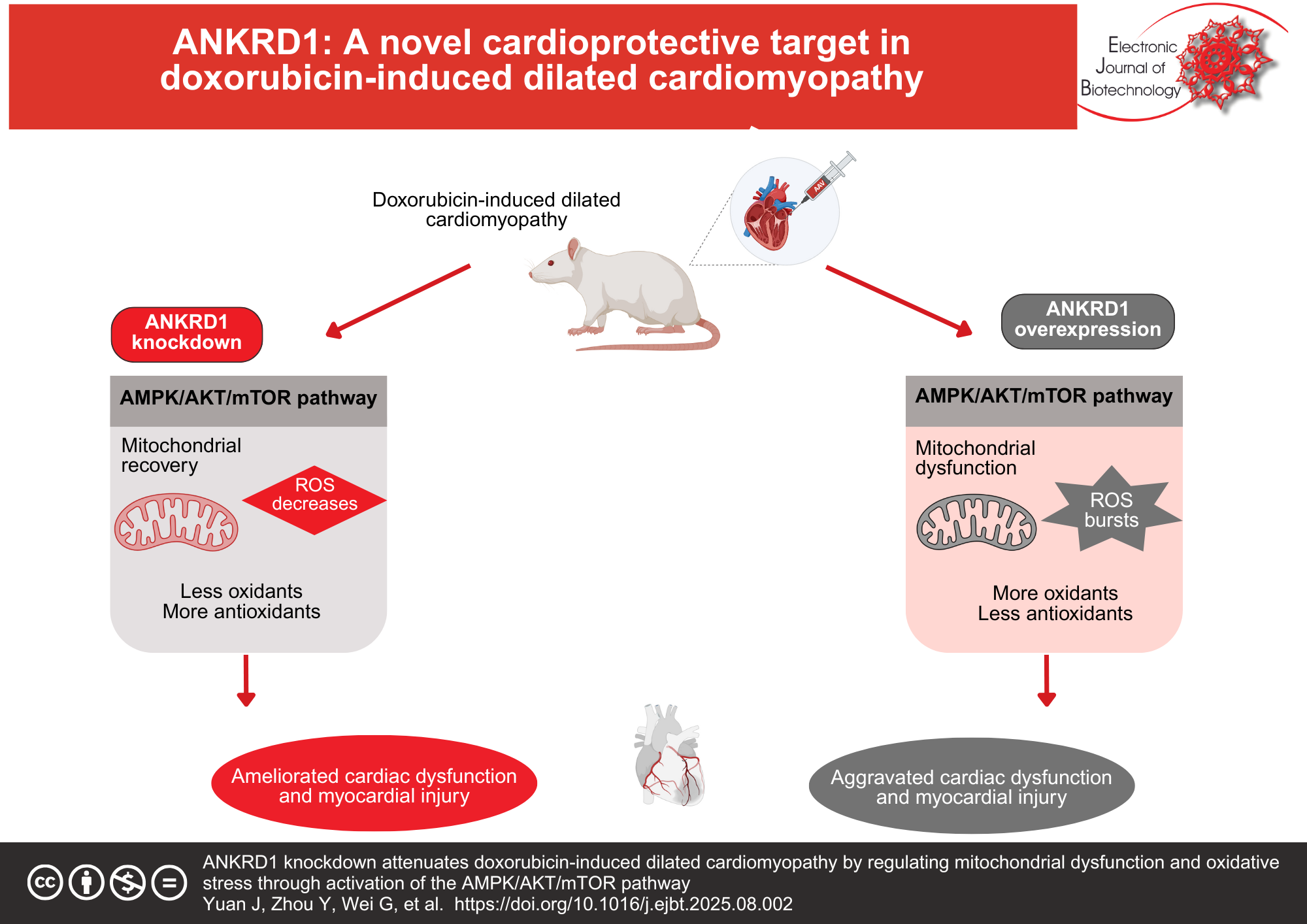
Results: DOX treatment significantly upregulated ANKRD1 expression in both rat models and H9c2 rat cardiomyocytes. In vivo, ANKRD1 knockdown ameliorated DOX-induced cardiac dysfunction, as demonstrated by improved left ventricular ejection fraction and fractional shortening, along with reduced serum levels of lactate dehydrogenase and creatine kinase-myocardial band. Conversely, ANKRD1 overexpression exacerbated cardiac impairment. Pathological examination revealed that ANKRD1 knockdown attenuated DOX-induced myocardial tissue damage and collagen deposition, while ANKRD1 overexpression intensified these pathological changes. Furthermore, ANKRD1 knockdown mitigated mitochondrial dysfunction and oxidative stress in DCM models both in vivo and in vitro. Mechanistically, ANKRD1 knockdown activated the AMP-activated protein kinase (AMPK)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling cascade, thereby attenuating DOX-induced cardiomyocyte toxicity, mitochondrial dysfunction, and oxidative stress. Rescue experiments using the AMPK inhibitor dorsomorphin confirmed this pathway’s involvement, as dorsomorphin treatment abolished the protective effects of ANKRD1 knockdown against DOX-induced cardiomyocyte damage.
Conclusions: ANKRD1 knockdown prevents DOX-induced DCM by ameliorating mitochondrial dysfunction and oxidative stress through activation of the AMPK/AKT/mTOR pathway. These findings establish ANKRD1 as a promising therapeutic target for preventing DOX-induced cardiotoxicity and DCM.
References
Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet 2017;390(10092):400-414. https://doi.org/10.1016/s0140-6736(16)31713-5. PMid: 28190577
Heymans, S., N.K. Lakdawala, C. Tschöpe, et al. Dilated cardiomyopathy: causes, mechanisms, and current and future treatment approaches. Lancet. 2023;402(10406):998-1011. https://doi.org/10.1016/s0140-6736(23)01241-2 PMid: 37716772
Reichart, D., C. Magnussen, T. Zeller, et al. Dilated cardiomyopathy: from epidemiologic to genetic phenotypes. A translational review of current literature. J Intern Med. 2019;286(4):362-372. https://doi.org/10.1111/joim.12944 PMid: 31132311
Orphanou, N., E. Papatheodorou, A. Anastasakis. Dilated cardiomyopathy in the era of precision medicine: latest concepts and developments. Heart Fail Rev. 2022;27(4):1173-1191. https://doi.org/10.1007/s10741-021-10139-0 PMid: 34263412
Harding, D., M.H.A. Chong, N. Lahoti, et al. Dilated cardiomyopathy and chronic cardiac inflammation: Pathogenesis, diagnosis and therapy. J Intern Med. 2023;293(1):23-47. https://doi.org/10.1111/joim.13556 PMid: 36030368
Kankeu, C., K. Clarke, E. Passante, et al. Doxorubicin-induced chronic dilated cardiomyopathy-the apoptosis hypothesis revisited. J Mol Med 2017;95(3):239-248. https://doi.org/10.1007/s00109-016-1494-0 PMid: 27933370
Sun, M., X. Zhang, B. Tan, et al. Potential role of endoplasmic reticulum stress in doxorubicin-induced cardiotoxicity-an update. Front Pharmacol 2024;15:1415108. https://doi.org/10.3389/fphar.2024.1415108 PMid: 39188945
Zhang, P., H. Lu, Y. Wu, et al. COX5A alleviates doxorubicin-induced cardiotoxicity by suppressing oxidative stress, mitochondrial dysfunction and cardiomyocyte apoptosis. Int J Mol Sci 2023;24(12):10400. https://doi.org/10.3390/ijms241210400 PMid: 37373547
Da Dalt, L., A.G. Cabodevilla, I.J. Goldberg, et al. Cardiac lipid metabolism, mitochondrial function, and heart failure. Cardiovasc Res. 2023;119(10):1905-1914. https://doi.org/10.1093/cvr/cvad100 PMid: 37392421
Chistiakov, D.A., T.P. Shkurat, A.A. Melnichenko, et al. The role of mitochondrial dysfunction in cardiovascular disease: a brief review. Ann Med. 2018;50(2):121-127. https://doi.org/10.1080/07853890.2017.1417631 PMid: 29237304
Huang, Y., W. Li, H. Sun, et al. Mitochondrial transfer in the progression and treatment of cardiac disease. Life Sci. 2024;358:123119. https://doi.org/10.1016/j.lfs.2024.123119 PMid: 39395616
Sharma, S., S. Bhattarai, H. Ara, et al. SOD2 deficiency in cardiomyocytes defines defective mitochondrial bioenergetics as a cause of lethal dilated cardiomyopathy. Redox Biol. 2020;37:101740. https://doi.org/10.1016/j.redox.2020.101740 PMid: 33049519
Li, E., X. Li, J. Huang, et al. BMAL1 regulates mitochondrial fission and mitophagy through mitochondrial protein BNIP3 and is critical in the development of dilated cardiomyopathy. Protein Cell. 2020;11(9):661-679. https://doi.org/10.1007/s13238-020-00713-x PMid: 32277346
Enomoto, H., N. Mittal, T. Inomata, et al. Dilated cardiomyopathy-linked heat shock protein family D member 1 mutations cause up-regulation of reactive oxygen species and autophagy through mitochondrial dysfunction. Cardiovasc Res. 2021;117(4):1118-1131. https://doi.org/10.1093/cvr/cvaa158 PMid: 32520982
Du, H., Y. Zhao, J. Wen, et al. LncRNA DCRT protects against dilated cardiomyopathy by preventing NDUFS2 alternative splicing by binding to PTBP1. Circulation 2024;150(13):1030-1049. https://doi.org/10.1161/circulationaha.123.067861 PMid: 38841852
Peoples, J.N., A. Saraf, N. Ghazal, et al. Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med 2019;51(12):1-13. https://doi.org/10.1038/s12276-019-0355-7 PMid: 31857574
Ramaccini, D., V. Montoya-Uribe, F.J. Aan, et al. mitochondrial function and dysfunction in dilated cardiomyopathy. Front Cell Dev Biol. 2020;8:624216. https://doi.org/10.3389/fcell.2020.624216 PMid: 33511136
Xie, S., Y. Sun, X. Zhao, et al. An update of the molecular mechanisms underlying anthracycline induced cardiotoxicity. Front Pharmacol. 2024;15:1406247. https://doi.org/10.3389/fphar.2024.1406247 PMid: 38989148
Han, Q., J. Shi, Y. Yu, et al. Calycosin alleviates ferroptosis and attenuates doxorubicin-induced myocardial injury via the Nrf2/SLC7A11/GPX4 signaling pathway. Front Pharmacol. 2024;15:1497733. https://doi.org/10.3389/fphar.2024.1497733 PMid: 39600362
Ye, J., Y. Huang, B. Que, et al. Interleukin-12p35 knock out aggravates doxorubicin-induced cardiac injury and dysfunction by aggravating the inflammatory response, oxidative stress, apoptosis and autophagy in mice. EBioMedicine. 2018;35:29-39. https://doi.org/10.1016/j.ebiom.2018.06.009 PMid: 30228093
Wang, Z., M. Wang, J. Liu, et al. Inhibition of TRPA1 attenuates doxorubicin-induced acute cardiotoxicity by suppressing oxidative stress, the inflammatory response, and endoplasmic reticulum stress. Oxid Med Cell Longev. 2018;2018(1):5179468. https://doi.org/10.1155/2018/5179468 PMid: 29682158
Moulik, M., M. Vatta, S.H. Witt, et al. ANKRD1, the gene encoding cardiac ankyrin repeat protein, is a novel dilated cardiomyopathy gene. J Am Coll Cardiol. 2009;54(4):325-33. https://doi.org/10.1016/j.jacc.2009.02.076 PMid: 19608030
Mastrototaro, G., P. Carullo, J. Zhang, et al. Ablation of palladin in adult heart causes dilated cardiomyopathy associated with intercalated disc abnormalities. Elife. 2023;12:e78629. https://doi.org/10.7554/eLife.78629 PMid: 36927816
Bogomolovas, J., K. Brohm, J. ?elutkien?, et al. Induction of Ankrd1 in dilated cardiomyopathy correlates with the heart failure progression. Biomed Res Int. 2015;2015(1):273936. https://doi.org/10.1155/2015/273936 PMid: 25961010
Ma, X., C. Mo, L. Huang, et al. Robust rank aggregation and least absolute shrinkage and selection operator analysis of novel gene signatures in dilated cardiomyopathy. Front Cardiovasc Med. 2021;8:747803. https://doi.org/10.3389/fcvm.2021.747803 PMid: 34970603
Piroddi, N., P. Pesce, B. Scellini, et al. Myocardial overexpression of ANKRD1 causes sinus venosus defects and progressive diastolic dysfunction. Cardiovasc Res. 2020;116(8):1458-1472. https://doi.org/10.1093/cvr/cvz291 PMid: 31688894
Murphy, N.P., E.R. Lubbers, P.J. Mohler. Advancing our understanding of AnkRD1 in cardiac development and disease. Cardiovasc Res. 2020;116(8):1402-1404. https://doi.org/10.1093/cvr/cvaa063 PMid: 32186710
Rink?nait?, I., E. Šimoli?nas, M. Alksn?, et al. Genetic ablation of Ankrd1 mitigates cardiac damage during experimental autoimmune myocarditis in mice. Biomolecules. 2022;12(12):1898. https://doi.org/10.3390/biom12121898 PMid: 36551326
Shen, L.J., S. Lu, Y.H. Zhou, et al. Developing a rat model of dilated cardiomyopathy with improved survival. J Zhejiang Univ Sci B. 2016;17(12):975-983. https://doi.org/10.1631/jzus.B1600257 PMid: 27921402
Pang, X.F., X. Lin, J.J. Du, et al. LTBP2 knockdown by siRNA reverses myocardial oxidative stress injury, fibrosis and remodelling during dilated cardiomyopathy. Acta Physiol (Oxf). 2020;228(3):e13377. https://doi.org/10.1111/apha.13377 PMid: 31512380
Zhu, M., Y. Chen, L. Cheng, et al. Calsyntenin-1 promotes doxorubicin-induced dilated cardiomyopathy in rats. Cardiovasc Drugs Ther. 2024;38(2):237-252. https://doi.org/10.1007/s10557-022-07389-x PMid: 36350487
Zhang, L., Y. Zhang, D. Chen, et al. Value of label-free ubiquitin-proteomic analysis on defining the protective mechanism of valsartan against doxorubicin-induced heart failure. Curr Cancer Drug Targets. 2025;25(7):795-805. https://doi.org/10.2174/0115680096341637241231111922 PMid: 39817389
Zhang, S., X. Wei, H. Zhang, et al. Doxorubicin downregulates autophagy to promote apoptosis-induced dilated cardiomyopathy via regulating the AMPK/mTOR pathway. Biomed Pharmacother. 2023;162:114691. https://doi.org/10.1016/j.biopha.2023.114691 PMid: 37060659
Shen, L., C. Chen, X. Wei, et al. Overexpression of ankyrin repeat domain 1 enhances cardiomyocyte apoptosis by promoting p53 activation and mitochondrial dysfunction in rodents. Clin Sci (Lond). 2015;128(10):665-678. https://doi.org/10.1042/cs20140586 PMid: 25511237
Zolk, O., M. Marx, E. Jäckel, et al. ?-adrenergic stimulation induces cardiac ankyrin repeat protein expression: involvement of protein kinase A and calmodulin-dependent kinase. Cardiovasc Res. 2003;59(3):563-572. https://doi.org/10.1016/s0008-6363(03)00476-0 PMid: 14499857
Chen, C., L. Shen, S. Cao, et al. Cytosolic CARP promotes angiotensin II- or pressure overload-induced cardiomyocyte hypertrophy through calcineurin accumulation. PLoS One. 2014;9(8):e104040. https://doi.org/10.1371/journal.pone.0104040 PMid: 25089522
Song, Y., J. Xu, Y. Li, et al. Cardiac ankyrin repeat protein attenuates cardiac hypertrophy by inhibition of ERK1/2 and TGF-? signaling pathways. PLoS One. 2012;7(12):e50436. https://doi.org/10.1371/journal.pone.0050436 PMid: 23227174
Xie, R., S. Yuan, G. Hu, et al. Nuclear AGO2 promotes myocardial remodeling by activating ANKRD1 transcription in failing hearts. Mol Ther. 2024;32(5):1578-1594. https://doi.org/10.1016/j.ymthe.2024.03.018 PMid: 38475992
Tomczyk, M.M., K.G. Cheung, B. Xiang, et al. Mitochondrial Sirtuin-3 (SIRT3) prevents doxorubicin-induced dilated cardiomyopathy by modulating protein acetylation and oxidative stress. Circ Heart Fail. 2022;15(5):e008547. https://doi.org/10.1161/circheartfailure.121.008547 PMid: 35418250
Shipra, M.K. Tembhre, M.P. Hote, et al. PGC-1? agonist rescues doxorubicin-induced cardiomyopathy by mitigating the oxidative stress and necroptosis. Antioxidants 2023;12(9):1720. https://doi.org/10.3390/antiox12091720 PMid: 37760023
Zhao, J., T. Yang, J. Yi, et al. AP39 through AMPK-ULK1-FUNDC1 pathway regulates mitophagy, inhibits pyroptosis, and improves doxorubicin-induced myocardial fibrosis. iScience. 2024;27(4):109321. https://doi.org/10.1016/j.isci.2024.109321 PMid: 38558936
Murphy E, Liu JC. Mitochondrial calcium and reactive oxygen species in cardiovascular disease. Cardiovasc Res. 2023;119(5):1105-1116. https://doi.org/10.1093/cvr/cvac134 PMid: 35986915
Rabinovitch, R.C., B. Samborska, B. Faubert, et al. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 2017;21(1):1-9. https://doi.org/10.1016/j.celrep.2017.09.026 PMid: 28978464
Gao, Y., D. Zhao, W.Z. Xie, et al. Rap1GAP mediates angiotensin II-induced cardiomyocyte hypertrophy by inhibiting autophagy and increasing oxidative stress. Oxid Med Cell Longev. 2021;2021(1):7848027. https://doi.org/10.1155/2021/7848027 PMid: 33936386
Lin SC, Hardie DG. AMPK: Sensing glucose as well as cellular energy status. Cell Metab. 2018;27(2):299-313. https://doi.org/10.1016/j.cmet.2017.10.009 PMid: 29153408
Wang, Y., D. Sun, Y. Chen, et al. Alkaloids of Delphinium grandiflorum and their implication to H2O2-induced cardiomyocytes injury. Bioorg Med Chem. 2021;37:116113. https://doi.org/10.1016/j.bmc.2021.116113 PMid: 33744825

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Copyright (c) 2026 Electronic Journal of Biotechnology