Abstract
Background: N6-methyladenosine (m6A) methylation plays a key role in osteosarcoma (OS) progression. This study aimed to elucidate the function and mechanism of methyltransferase 16 (METTL16), an m6A methyltransferase, in OS progression.
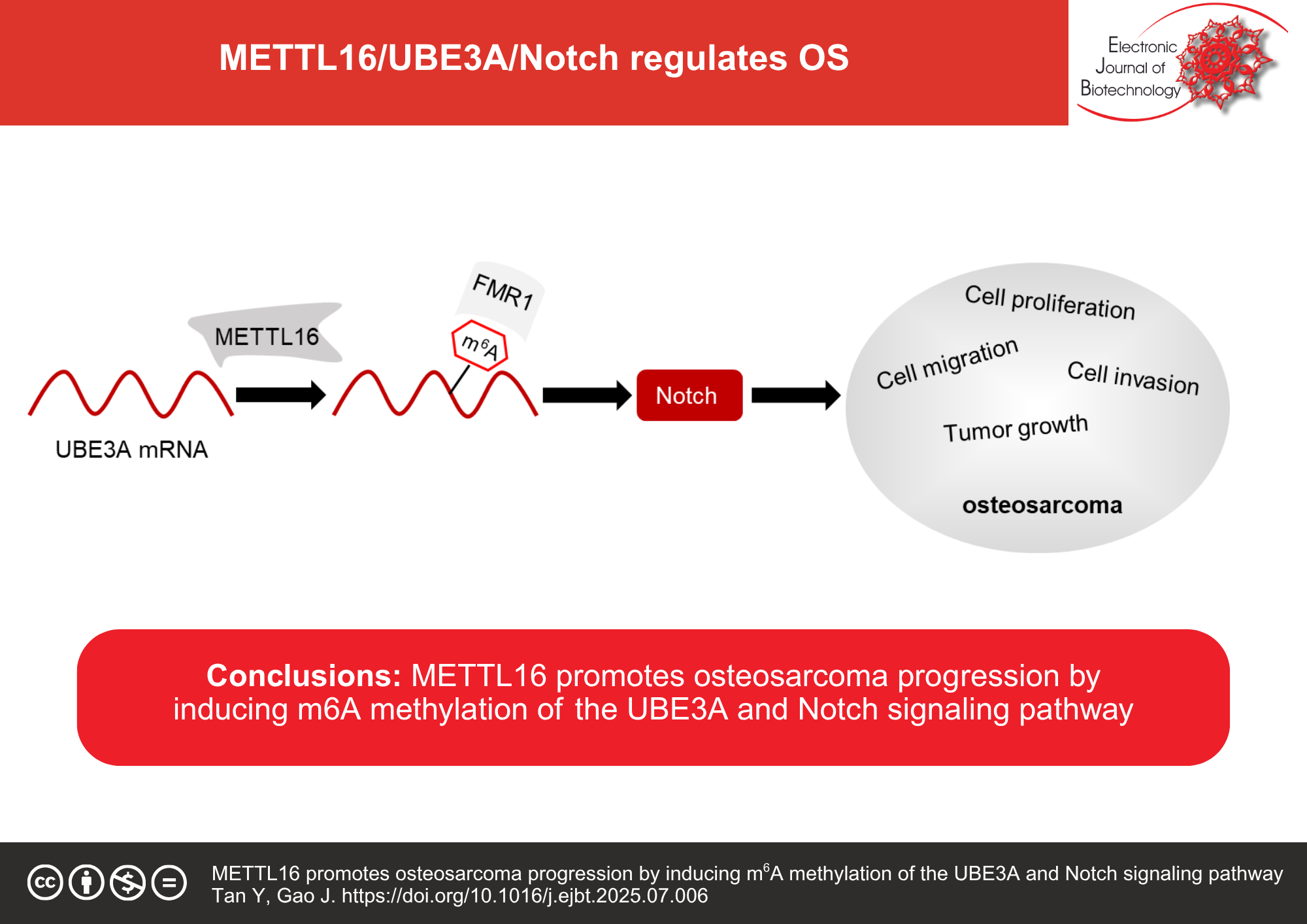
Results: Bioinformatics analysis with quantitative reverse-transcription polymerase chain reaction (qRT-PCR) revealed high METTL16 expression in OS. After performing cell functional experiments, METTL16 silencing was shown to decrease the proliferation, migration, and invasion of OS cells. Using qRT-PCR, methylated RNA immunoprecipitation quantitative polymerase chain reaction (MeRIP-qPCR), Western blotting, luciferase, RNA-binding protein immunoprecipitation (RIP), and RNA stability assays, METTL16 induced the m6A methylation of ubiquitin protein ligase E3A (UBE3A) to promote UBE3A expression and mRNA stability in OS cells in a fragile X messenger ribonucleoprotein 1 (FMR1)-dependent manner. Moreover, in vitro and in vivo results showed that UBE3A activated the Notch signaling pathway, thereby promoting OS cell malignancy. METTL16 knockdown partly reversed the oncogenic role of UBE3A in OS cells.
Conclusions: METTL16 acts as a tumor promotor in OS progression by modulating UBE3A expression via m6A methylation to activate the Notch signaling pathway. The findings highlight the therapeutic potential of disrupting the METTL16–UBE3A–Notch pathway axis in OS.
References
Kim C, Davis LE, Albert CM, et al. Osteosarcoma in pediatric and adult populations: Are adults just big kids? Cancers 2023;15(20):5044. http://doi.org/10.3390/cancers15205044 PMid: 37894411
Yu S, Yao X. Advances on immunotherapy for osteosarcoma. Mol Cancer. 2024;23(1):192. http://doi.org/10.1186/s12943-024-02105-9. PMid: 39245737
Harris MA, Hawkins CJ. Recent and ongoing research into metastatic osteosarcoma treatments. Int J Mol Sci. 2022;23(7):3817. http://doi.org/10.3390/ijms23073817. PMid: 35409176
Lilienthal I, Herold N. Targeting molecular mechanisms underlying treatment efficacy and resistance in osteosarcoma: A review of current and future strategies. Int J Mol Sci. 2020;21(18):6885. http://doi.org/10.3390/ijms21186885 PMid: 32961800
Sheng G, Gao Y, Yang Y, et al. Osteosarcoma and metastasis. Front Oncol. 2021;11:780264. http://doi.org/10.3389/fonc.2021.780264 PMid: 34956899
Moukengue B, Lallier M, Marchandet L, et al. Origin and therapies of osteosarcoma. Cancers 2022;14(14):3503. http://doi.org/10.3390/cancers14143503 PMid: 35884563
Uddin MB, Wang Z, Yang C. The m6A RNA methylation regulates oncogenic signaling pathways driving cell malignant transformation and carcinogenesis. Mol Cancer. 2021;20(1):61. http://doi.org/10.1186/s12943-021-01356-0 PMid: 33814008
Wang Y, Wang Y, Patel H, et al. Epigenetic modification of m6A regulator proteins in cancer. Mol Cancer. 2023;22(1):102. http://doi.org/10.1186/s12943-023-01810-1 PMid: 37391814
He PC, He C. m6A RNA methylation: from mechanisms to therapeutic potential. EMBO J. 2021;40(3):e105977. http://doi.org/10.15252/embj.2020105977 PMid: 33470439
Wang S, Lv W, Li T, et al. Dynamic regulation and functions of mRNA m6A modification. Cancer Cell Int. 2022;22(1):48. http://doi.org/10.1186/s12935-022-02452-x PMid: 35093087
Zhang J, Tong L, Liu Y, et al. The regulatory role of m6A modification in the maintenance and differentiation of embryonic stem cells. Genes Dis. 2024;11(5):101199 http://doi.org/10.1016/j.gendis.2023.101199 PMid: 38947741
Petri BJ, Klinge CM. m6A readers, writers, erasers, and the m6A epitranscriptome in breast cancer. J Mol Endocrinol 2023;70(2):e220110. http://doi.org/10.1530/JME-22-0110 PMid: 36367225
Yao Y, Liu P, Li Y, et al. Regulatory role of m6A epitranscriptomic modifications in normal development and congenital malformations during embryogenesis. Biomed Pharmacother. 2024;173:116171. http://doi.org/10.1016/j.biopha.2024.116171 PMid: 38394844
Ye F, Wu J, Zhang F. METTL16 epigenetically enhances GPX4 expression via m6A modification to promote breast cancer progression by inhibiting ferroptosis. Biochem Biophys Res Commun. 2023;638:1-6. http://doi.org/10.1016/j.bbrc.2022.10.065 PMid: 36434904
Yi T, Wang C, Ye X, et al. METTL16 inhibits pancreatic cancer proliferation and metastasis by promoting MROH8 RNA stability and inhibiting CAPN2 expression - experimental studies. Int J Surg. 2024;110(12):7701-7719. http://doi.org/10.1097/JS9.0000000000002116 PMid: 39434688
Chaudhary P, Proulx J, Park IW. Ubiquitin-protein ligase E3A (UBE3A) mediation of viral infection and human diseases. Virus Res. 2023;335:199191. http://doi.org/10.1016/j.virusres.2023.199191 PMid: 37541588
Zhu J, Tsai NP. Ubiquitination and E3 ubiquitin ligases in rare neurological diseases with comorbid epilepsy. Neuroscience. 2020;428:90-99. http://doi.org/10.1016/j.neuroscience.2019.12.030 PMid: 31931110
Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation - lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18(7):401-417. http://doi.org/10.1038/s41571-021-00479-z PMid: 33654306
Zhang N, Shen J, Gou L, et al. UBE3A deletion enhances the efficiency of immunotherapy in non-small-cell lung cancer. Bioengineered. 2022;13(5):11577-11592. http://doi.org/10.1080/21655979.2022.2069328 PMid: 35531878
Zheng Z, Zhang B, Yu H, et al. UBE3A activates the NOTCH pathway and promotes esophageal cancer progression by degradation of ZNF185. Int J Biol Sci. 2021;17(12):3024-3035. http://doi.org/10.7150/ijbs.61117 PMid: 34421347
Isakoff MS, Bielack SS, Meltzer P, et al. Osteosarcoma: Current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33(27):3029-3035. http://doi.org/10.1200/JCO.2014.59.4895 PMid: 26304877
Tian H, Cao J, Li B, et al. Managing the immune microenvironment of osteosarcoma: the outlook for osteosarcoma treatment. Bone Res. 2023;11(1):11. http://doi.org/10.1038/s41413-023-00246-z PMid: 36849442
Wang XK, Zhang YW, Wang CM, et al. METTL16 promotes cell proliferation by up-regulating cyclin D1 expression in gastric cancer. J Cell Mol Med. 2021;25(14):6602-6617. http://doi.org/10.1111/jcmm.16664 PMid: 34075693
Li Q, Wang Y, Meng X, et al. METTL16 inhibits papillary thyroid cancer tumorigenicity through m6A/YTHDC2/SCD1-regulated lipid metabolism. Cell Mol Life Sci. 2024;81(1):81. http://doi.org/10.1007/s00018-024-05146-x PMid: 38334797
Cheng J, Xu Z, Tan W, et al. METTL16 promotes osteosarcoma progression by downregulating VPS33B in an m6A-dependent manner. J Cell Physiol. 2024;239(3):e31068. http://doi.org/10.1002/jcp.31068 PMid: 37357526
Shi Q, Xue C, Zeng Y, et al. Notch signaling pathway in cancer: from mechanistic insights to targeted therapies. Signal Transduct Target Ther. 2024;9(1):128. http://doi.org/10.1038/s41392-024-01828-x PMid: 38797752
Li X, Yan X, Wang Y, et al. The Notch signaling pathway: A potential target for cancer immunotherapy. J Hematol Oncol. 2023;16(1):45. http://doi.org/10.1186/s13045-023-01439-z PMid: 37131214
Mashanov V, Akiona J, Khoury M, et al. Active Notch signaling is required for arm regeneration in a brittle star. PLoS One. 2020;15(5):e0232981. http://doi.org/10.1371/journal.pone.0232981 PMid: 32396580
Zhan P, Lu Y, Lu J, et al. The activation of the Notch signaling pathway by UBE2C promotes the proliferation and metastasis of hepatocellular carcinoma. Sci Rep. 2024;14(1):22859. http://doi.org/10.1038/s41598-024-72714-3. PMid: 39353974
Jiang N, Hu Y, Wang M, et al. The Notch signaling pathway contributes to angiogenesis and tumor immunity in breast cancer. Breast Cancer (Dove Med Press). 2022;14:291-309. http://doi.org/10.2147/BCTT.S376873 PMid: 36193236
Zhang T, Chen S, Peng Y, et al. NOVA1-Mediated SORBS2 isoform promotes colorectal cancer migration by activating the Notch pathway. Front Cell Dev Biol. 2021;9:673873. http://doi.org/10.3389/fcell.2021.673873 PMid: 34692669
Ong KOK, Mok MMH, Niibori-Nambu A, et al. Activation of NOTCH signaling impedes cell proliferation and survival in acute megakaryoblastic leukemia. Exp Hematol. 2024;137:104255. http://doi.org/10.1016/j.exphem.2024.104255 PMid: 38876252
Cheng J, Zhang Y, Wan R, et al. CEMIP promotes osteosarcoma progression and metastasis through activating Notch signaling pathway. Front Oncol. 2022;12:919108. http://doi.org/10.3389/fonc.2022.919108 PMid: 35957875
Zhang X, Bian H, Wei W, et al. DLX5 promotes osteosarcoma progression via activation of the NOTCH signaling pathway. Am J Cancer Res. 2021;11(6):3354-3374. PMid: 34249467
Liu P, Man Y, Wang Y, et al. Mechanism of BMP9 promotes growth of osteosarcoma mediated by the Notch signaling pathway. Oncol Lett. 2016;11(2):1367-1370. http://doi.org/10.3892/ol.2015.4067 PMid: 26893744

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Copyright (c) 2025 Electronic Journal of Biotechnology