Abstract
Background: Banana plants possess numerous medicinal properties due to the presence of various phytochemicals. This study aimed to assess the phytochemical profile of the crude extracts of leaf, pseudostem, and corm parts of selected banana cultivars via standard techniques and thin-layer chromatography (TLC) and to evaluate their antimicrobial activities against several food-borne and clinically important human pathogens, including two Gram-positive bacteria, six Gram-negative bacteria, and four yeasts.
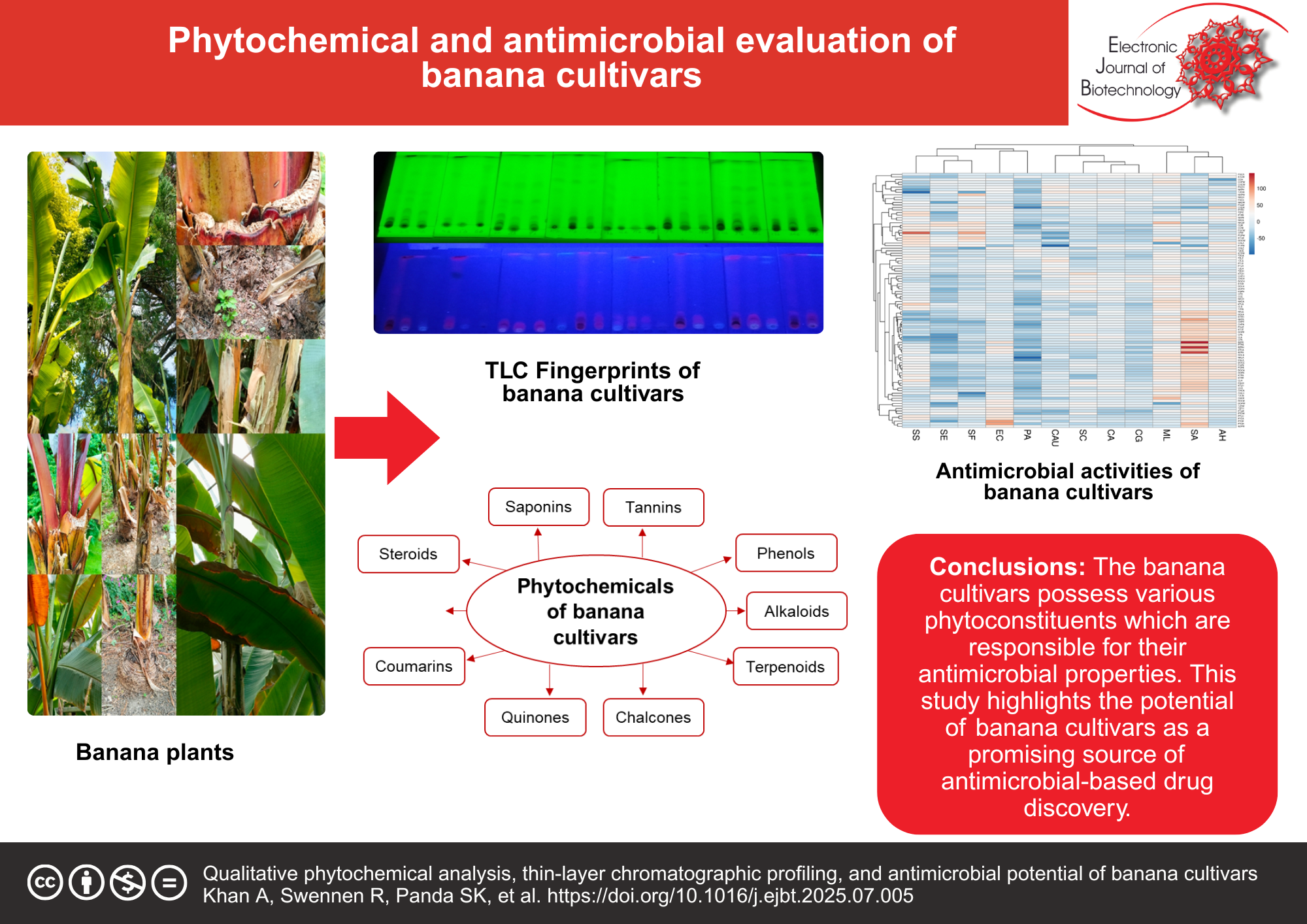
Results: The results demonstrated that the Cachaco (41%), Tereza (38%), Fougamou (30%), Pelipita (28%), Giant Cavendish (26%), and Kluai Teparot (26%) cultivars presented significant antimicrobial activity against pathogens compared with Dole (24%), Namwah Khom (20%), and Mbwazirume (16%) cultivars. Moreover, the leaves (40%) of cultivars extracted in water (61%) and acetone (55%) yielded the most active antimicrobial extracts compared with the pseudostem (33%) and corm (26%) extracts prepared in ethanol (38%) or hexane (28%). Overall, the antimicrobial activities with the lowest 50% inhibitory concentration (IC50) values, especially those with values less than 200 µg/mL for bacteria and 100 µg/mL for yeasts, were reported in the leaves of Cachaco and Giant Cavendish, followed by different parts of Tereza, Pelipita, and other banana cultivars. Phytochemical analysis and TLC profiling confirmed the presence of various groups of phytochemicals in the extracts of the selected banana cultivars.
Conclusions: This study revealed that the Cachaco, Giant Cavendish, Pelipita, and Tereza cultivars possess significant antimicrobial activity, warranting further bioassay-guided antimicrobial studies for the isolation and identification of bioactive compounds, which could be useful as novel drug candidates with the highest potency.
References
Swargiary A, Boro H, Roy MK, et al. Phytochemistry and pharmacological property of Musa balbisiana Colla: A Mini-Review. Pharmacogn Rev 2021;15(29):91-5. https://doi.org/10.5530/phrev.2021.15.11
Çelebi Ö, Fidan H, Iliev I, et al. Chemical composition, biological activities, and surface tension properties of Melissa officinalis L. essential oil. Turkish Journal of Agriculture and Forestry 2023;47(1):67–78. https://doi.org/10.55730/1300-011X.3065
Fidan H, Stankov S, Petkova N, et al. Evaluation of chemical composition, antioxidant potential and functional properties of carob (Ceratonia siliqua L.) seeds. J Food Sci Technol 2020;57:2404–13. https://doi.org/10.1007/s13197-020-04274-z PMid: 32549590
Atanasov AG, Waltenberger B, Pferschy-Wenzig E-M, et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol Adv 2015;33(8):1582–614. https://doi.org/10.1016/j.biotechadv.2015.08.001 PMid: 26281720
Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites 2012;2(2):303–36. https://doi.org/10.3390/metabo2020303 PMid: 24957513
Ji H, Li X, Zhang H. Natural products and drug discovery: Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep 2009;10:194–200. https://doi.org/10.1038/embor.2009.12 PMid: 19229284
Rodríguez-Negrete EV, Morales-González Á, Madrigal-Santillán EO, et al. Phytochemicals and their usefulness in the maintenance of health. Plants 2024;13(4):523. https://doi.org/10.3390/plants13040523 PMid: 38498532
Zia-Ul-Haq M, Ahmad S, Qayum M, et al. Compositional studies and antioxidant potential of Albizia lebbeck (L.) Benth. pods and seeds. Turkish Journal of Biology 2013;37:25–32. https://doi.org/10.3906/biy-1204-38
De Langhe E, Vrydaghs L, Perrier X, et al. Fahien reconsidered: Pleistocene exploitation of wild bananas and Holocene introduction of Musa cultivars to Sri Lanka. J Quat Sci 2019;34(6):405–9. https://doi.org/10.1002/jqs.3108
Sulaymanov K, Johri P, Azimov A, et al. Phytochemical analysis of turmeric (Curcuma longa L.) grown in Uzbekistan. Turkish Journal of Agriculture and Forestry 2024;48(5):647–57. https://doi.org/10.55730/1300-011X.3209
Probojati RT, Listyorini D, Sulisetijono S, et al. Phylogeny and estimated genetic divergence times of banana cultivars (Musa spp.) from Java Island by maturase K (matK) genes. Bull Natl Res Cent 2021;45:33. https://doi.org/10.1186/s42269-021-00492-3
Amah D, van Biljon A, Brown A, et al. Recent advances in banana (Musa spp.) biofortification to alleviate vitamin A deficiency. Crit Rev Food Sci Nutr 2019;59(21):3498–510. https://doi.org/10.1080/10408398.2018.1495175 PMid: 29999424
Martin G, Cardi C, Sarah G, et al. Genome ancestry mosaics reveal multiple and cryptic contributors to cultivated banana. The Plant Journal 2020;102(5):1008–25. https://doi.org/10.1111/tpj.14683 PMid: 31930580
de Jesus ON, Silva S de O e, Amorim EP, et al. Genetic diversity and population structure of Musa accessions in ex situ conservation. BMC Plant Biol 2013;13:41. https://doi.org/10.1186/1471-2229-13-41 PMid: 23497122
Panda SK, Castro AHF, Jouneghani RS, et al. Antiviral and cytotoxic activity of different plant parts of banana (Musa spp.). Viruses 2020;12(5):549. https://doi.org/10.3390/v12050549 PMid: 32429324
Li X, Yu S, Cheng Z, et al. Origin and evolution of the triploid cultivated banana genome. Nat Genet 2024;56:136–42. https://doi.org/10.1038/s41588-023-01589-3 PMid: 38082204
Sardos J, Cenci A, Martin G, et al. Painting the diversity of a world’s favorite fruit: A next generation catalog of cultivated bananas. Plants, People, Planet 2025;7(1):263–83. https://doi.org/10.1002/ppp3.10581
FAOSTAT. Crop production, statistics division. Food and Agriculture Organization of the United Nations 2019.
Mathew NS, Negi PS. Traditional uses, phytochemistry and pharmacology of wild banana (Musa acuminata Colla): A review. J Ethnopharmacol 2017;196:124–40. https://doi.org/10.1016/j.jep.2016.12.009 PMid: 27988402
Padam BS, Tin HS, Chye FY, et al. Banana by-products: An under-utilized renewable food biomass with great potential. J Food Sci Technol 2014;51:3527–45. https://doi.org/10.1007/s13197-012-0861-2 PMid: 25477622
Kora AJ. Leaves as dining plates, food wraps and food packing material: Importance of renewable resources in Indian culture. Bull Natl Res Cent 2019;43:205. https://doi.org/10.1186/s42269-019-0231-6
Shaikh JR, Patil M. Qualitative tests for preliminary phytochemical screening: An overview. Int J Chem Stud 2020;8(2):603–8. https://doi.org/10.22271/chemi.2020.v8.i2i.8834
Hu H, Tekin V, Hu B, et al. Metabolic profiling of Chimonanthus grammatus via UHPLC-HRMS-MS with computer-assisted structure elucidation and its antimicrobial activity. Front Plant Sci 2023;14:1138913. https://doi.org/10.3389/fpls.2023.1138913 PMid: 37229132
Shrestha D, Magar AB, Pakka S, et al. Phytochemical analysis, antioxidant, antimicrobial, and toxicity studies of Schima wallichii growing in Nepal. Int J Food Prop 2024;27:273–85. https://doi.org/10.1080/10942912.2024.2304267
Kagan IA, Flythe MD. Thin-layer chromatographic (TLC) separations and bioassays of plant extracts to identify antimicrobial compounds. J Vis Exp 2014;85:e51411. https://doi.org/10.3791/51411 PMid: 24747583
Zahiruddin S, Parveen A, Khan W, et al. TLC?based metabolite profiling and bioactivity?based scientific validation for use of water extracts in AYUSH formulations. Evidence?Based Complementary and Alternative Medicine 2021;2021(1):2847440. https://doi.org/10.1155/2021/2847440 PMid: 35003294
Jouneghani RS, Castro AHF, Panda SK, et al. Antimicrobial activity of selected banana cultivars against important human pathogens, including candida biofilm. Foods 2020;9(4):435. https://doi.org/10.3390/foods9040435 PMid: 32260420
Panda SK, Mohanta YK, Padhi L, et al. Antimicrobial activity of select edible plants from Odisha, India against food-borne pathogens. LWT 2019;113:108246. https://doi.org/10.1016/j.lwt.2019.06.013
Alemu M, Lulekal E, Asfaw Z, et al. Antibacterial activity and phytochemical screening of traditional medicinal plants most preferred for treating infectious diseases in Habru District, North Wollo Zone, Amhara Region, Ethiopia. PLoS One 2024;19:e0300060. https://doi.org/10.1371/journal.pone.0300060 PMid: 38442129
Asfaw A, Lulekal E, Bekele T, et al. Antibacterial and phytochemical analysis of traditional medicinal plants: an alternative therapeutic approach to conventional antibiotics. Heliyon 2023;9(11):e22462. https://doi.org/10.1016/j.heliyon.2023.e22462 PMid: 38045177
Dubale S, Kebebe D, Zeynudin A, et al. Phytochemical screening and antimicrobial activity evaluation of selected medicinal plants in Ethiopia. J Exp Pharmacol 2023;2023(15):51–62. https://doi.org/10.2147/JEP.S379805 PMid: 36789235
Akhtar MS, Rafiullah M, Shehata WA, et al. Comparative phytochemical, thin layer chromatographic profiling and antioxidant activity of extracts from some Indian herbal drugs. Journal of Bioresources and Bioproducts 2022;7(2):128–34. https://doi.org/10.1016/j.jobab.2022.01.001
Panda SK, Das R, Leyssen P, et al. Assessing medicinal plants traditionally used in the Chirang Reserve Forest, Northeast India for antimicrobial activity. J Ethnopharmacol 2018;225:220–33. https://doi.org/10.1016/j.jep.2018.07.011 PMid: 30005956
Hu H, Hu C, Peng J, et al. Bioassay-guided interpretation of antimicrobial compounds in Kumu, a TCM preparation from Picrasma quassioides’ stem via UHPLC-Orbitrap-ion trap mass spectrometry combined with fragmentation and retention time calculation. Front Pharmacol 2021;12:761751. https://doi.org/10.3389/fphar.2021.761751 PMid: 34776978
Panda SK, Padhi L, Leyssen P, et al. Antimicrobial, anthelmintic, and antiviral activity of plants traditionally used for treating infectious disease in the Similipal Biosphere Reserve, Odisha, India. Front Pharmacol 2017;8:658. https://doi.org/10.3389/fphar.2017.00658 PMid: 29109684
Esonu CE, Iheme CI, Mjoku OC, et al. Investigation of proximate composition and bioactive components in banana (Musa acuminata) peels using advanced analytical techniques. Nutrition and Food Processing 2024;7(10):256. https://doi.org/10.31579/2637-8914/256
Harith SS, Yasim NH, Harun A, Omar WS. Phytochemical screening, antifungal and antibacterial activities of Musa acuminata plant. Malaysian Journal of Analytical Sciences 2018;22(3):452–7. https://doi.org/10.17576/mjas-2018-2203-11
Onyema C, Ofor C, Okudo V, et al. Phytochemical and antimicrobial analysis of banana pseudo stem (Musa acuminata). Br J Pharm Res 2016;10(1):1–9. https://doi.org/10.9734/BJPR/2016/22593
Ehiowemwenguan G, Emoghene AO, Inetianbor JE. Antibacterial and phytochemical analysis of Banana fruit peel. IOSR J Pharm 2014;4(8):18–25. https://doi.org/10.9790/3013-0408018025
Adil M, Filimban FZ, Ambrin, et al. Phytochemical screening, HPLC analysis, antimicrobial and antioxidant effect of Euphorbia parviflora L. (Euphorbiaceae Juss.). Sci Rep 2024;14:5627. https://doi.org/10.1038/s41598-024-55905-w PMid: 38454096
Ozyigit II, Dogan I, Hocaoglu-Ozyigit A, et al. Production of secondary metabolites using tissue culture-based biotechnological applications. Front Plant Sci 2023;14:1132555. https://doi.org/10.3389/fpls.2023.1132555 PMid: 37457343
Pang Z, Chen J, Wang T, et al. Linking plant secondary metabolites and plant microbiomes: a review. Front Plant Sci 2021;12:621276. https://doi.org/10.3389/fpls.2021.621276 PMid: 33737943
Singh PK, Singh J, Medhi T, et al. Phytochemical screening, quantification, FT-IR analysis, and in silico characterization of potential bio-active compounds identified in HR-LC/MS analysis of the polyherbal formulation from Northeast India. ACS Omega 2022;7(37):33067–78. https://doi.org/10.1021/acsomega.2c03117 PMid: 36157760
Marston A. Thin-layer chromatography with biological detection in phytochemistry. J Chromatogr A 2011;1218(18):2676–83. https://doi.org/10.1016/j.chroma.2010.12.068 PMid: 21236438
Tsamo CVP, Herent M-F, Tomekpe K, et al. Effect of boiling on phenolic profiles determined using HPLC/ESI-LTQ-Orbitrap-MS, physico-chemical parameters of six plantain banana cultivars (Musa sp). Journal of Food Composition and Analysis 2015;44:158–69. https://doi.org/10.1016/j.jfca.2015.08.012
Pereira A, Maraschin M. Banana (Musa spp) from peel to pulp: Ethnopharmacology, source of bioactive compounds and its relevance for human health. J Ethnopharmacol 2015;160:149–63. https://doi.org/10.1016/j.jep.2014.11.008 PMid: 25449450
Vu HT, Scarlett CJ, Vuong QV. Phenolic compounds within banana peel and their potential uses: A review. J Funct Foods 2018;40:238–48. https://doi.org/10.1016/j.jff.2017.11.006
Asuquo EG, Udobi CE. Antibacterial and toxicity studies of the ethanol extract of Musa paradisiaca leaf. Cogent Biol 2016;2(1):1219248. https://doi.org/10.1080/23312025.2016.1219248
Mokbel MS, Hashinaga F. Antibacterial and antioxidant activities of banana (Musa, AAA cv. Cavendish) fruits peel. Am J Biochem Biotechnol 2005;1(3):125–31. https://doi.org/10.3844/ajbbsp.2005.125.131
Richter ER, Vore LA. Antimicrobial activity of banana puree. Food Microbiol 1989;6(3):179–87. https://doi.org/10.1016/S0740-0020(89)80026-7
Ahmad I, Beg AZ. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol 2001;74(2):113–23. https://doi.org/10.1016/S0378-8741(00)00335-4 PMid: 11167029
Alisi CS, Nwanyanwu CE, Akujobi CO, et al. Inhibition of dehydrogenase activity in pathogenic bacteria isolates by aqueous extracts of Musa paradisiaca (Var Sapientum). Afr J Biotechnol 2008;7(12):1821-5. https://doi.org/10.5897/AJB2008.000-5029
Kapadia SP, Pudakalkatti PS, Shivanaikar S. Detection of antimicrobial activity of banana peel (Musa paradisiaca L.) on Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans: An in vitro study. Contemp Clin Dent 2015;6(4):496–9. https://doi.org/10.4103/0976-237X.169864 PMid: 26681854
Siddique S, Nawaz S, Muhammad F, et al. Phytochemical screening and in-vitro evaluation of pharmacological activities of peels of Musa sapientum and Carica papaya fruit. Nat Prod Res 2018;32(11):1333–6. https://doi.org/10.1080/14786419.2017.1342089 PMid: 28627245

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Copyright (c) 2025 Electronic Journal of Biotechnology