Abstract
Background: This study aims to investigate the ferroptosis-inducing effects of Tianma Granules (TMGs) in colorectal cancer and elucidate its molecular mechanisms. Ferroptosis, an iron-dependent form of regulated cell death, represents a novel therapeutic target for cancer. We combined network pharmacology with experimental validation to explore TMG’s anti-cancer potential through ferroptosis modulation.
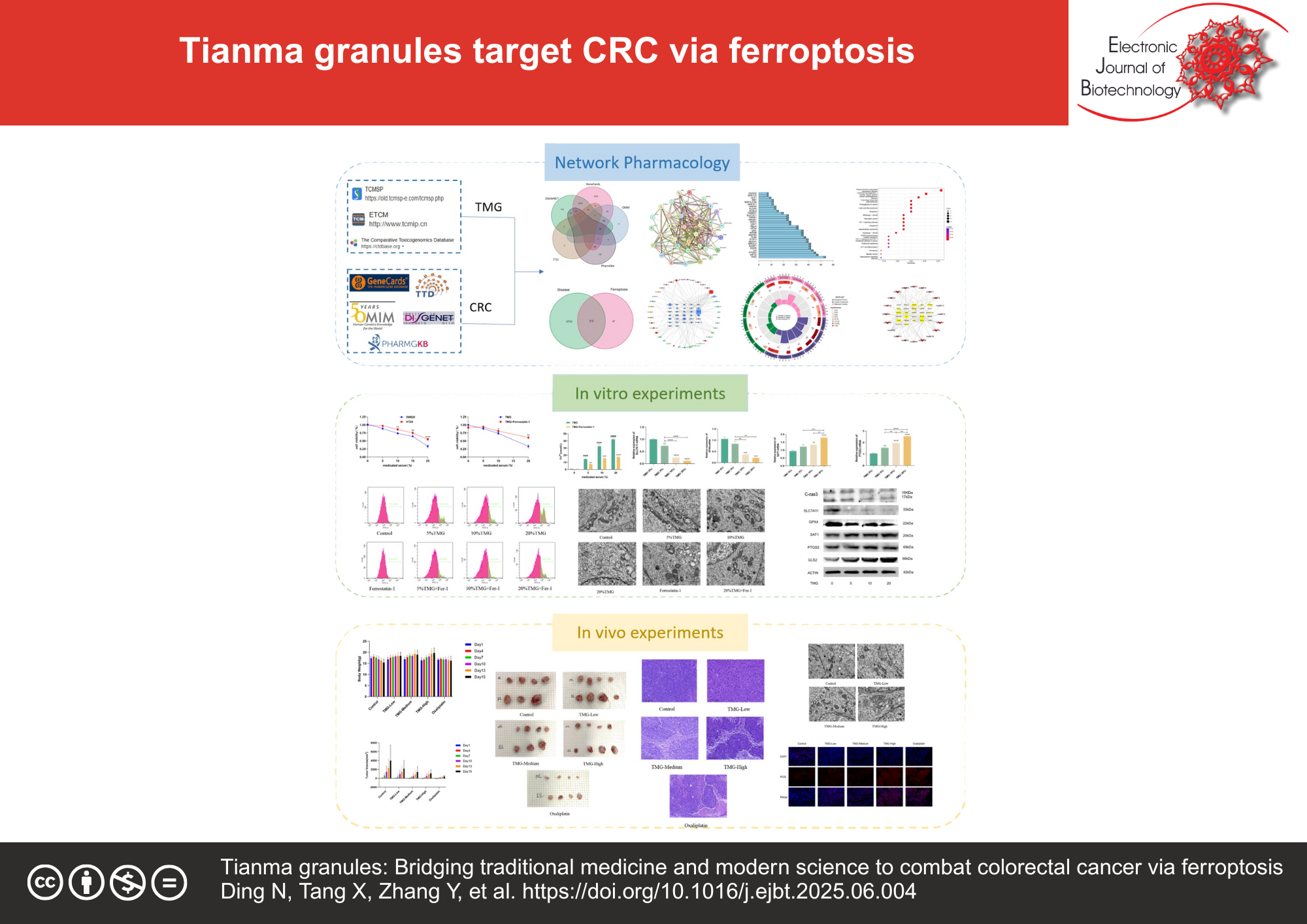
Results: Network pharmacology identified 382 ferroptosis-related genes overlapping with 12,944 CRC-associated targets (p < 0.05), with SLC7A11, GPX4, SAT1, PTGS2, and GLS2 prioritized as core targets. In vitro, TMG dose-dependently suppressed CRC cell proliferation (p < 0.05), elevated reactive oxygen species (p < 0.05) and ferrous ion levels (p < 0.01), effects reversed by ferroptosis inhibitor, Ferrostatin-1. c-Casp3 levels were unchanged (p > 0.05), excluding apoptosis. Transmission electron microscopy revealed mitochondrial cristae fragmentation and vacuolation, hallmark features of ferroptosis. Molecular analyses demonstrated TMG-mediated downregulation of SLC7A11 and GPX4, alongside upregulation of SAT1, PTGS2, and GLS2 (p < 0.05). In xenograft models, high-dose TMG (23.2 g/kg) reduced tumor volume, attenuated cachexia, and elevated intratumoral ROS and Fe2+ levels (p < 0.01), corroborating ferroptosis induction in vivo.
Conclusions: TMG suppresses CRC progression by inducing ferroptosis via dual inhibition of SLC7A11/GPX4 and activation of SAT1/PTGS2/GLS2. This study bridges traditional medicine and ferroptosis biology, positioning TMG as a novel therapeutic candidate for CRC.
References
Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.CA Cancer J Clin 2024;74(3):229-263. https://doi.org/10.3322/caac.21834 PMid: 38572751
Salva de Torres C, Baraibar I, Saoudi Gonzalez N, et al. Current and emerging treatment paradigms in colorectal cancer: Integrating hallmarks of cancer. Int J Mol Sci 2024;25(13):6967. https://doi.org/10.3390/ijms25136967 PMid: 39000083
Adebayo A, Agbaje K, Adesina SK, et al. Colorectal cancer: Disease process, current treatment options, and future perspectives. Pharmaceutics 2023;15(11):2620. https://doi.org/10.3390/pharmaceutics15112620 PMid: 38004598
Peng TS, Xie B, He YH, et al. Clinical effect of Tianma granules in inhibiting tumor recurrence after colorectal cancer operation. Pharmacology and Clinics of Chinese Materia Medica 2016;32(03):191-193.
Sun Y, Wang MH, Xiao Y, et al. Effect of Tianma Granule on expression of RAGE, CD34 and D2-40 in colon cancer transplanted tumor tissue of nude mice. Clinical Journal of Traditional Chinese Medicine 2023,35(12):2377-2381.
Yao X, Yuan WJ, He YH, et al. Study on the regulation of Tianma granules by TLR4/NF-?B/Klotho pathway of colorectal cancer transplanted in nude mice. Asia-Pacific Traditional Medicine 2024;20(02):11-14.
Tang XJ, He M, Ren Y, et al. Traditional Chinese Medicine formulas-based interventions on colorectal carcinoma prevention: The efficacies, mechanisms and advantages. .J Ethnopharmacol 2025;337 (part 3):119008. https://doi.org/10.1016/j.jep.2024.119008 PMid: 39471879
Nogales C, Mamdouh ZM, List M, et al. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci 2022;43(2):136-150. https://doi.org/10.1016/j.tips.2021.11.004 PMid: 34895945
Zheng SH, Liang YJ, Xue TY, et al. Application of network pharmacology in traditional Chinese medicine for the treatment of digestive system diseases. Front Pharmacol 2024;15:1412997. https://doi.org/10.3389/fphar.2024.1412997 PMid: 39086391
Luo TT, Lu Y, Yan SK, et al. et al. Network pharmacology in research of Chinese Medicine Formula: Methodology, application and prospective. Chin J Integr Med 2020;26:72-80. https://doi.org/10.1007/s11655-019-3064-0 PMid: 30941682
Wang X, Ren XX, Xu L, et al. Recent progress of ferroptosis in cancers and drug discovery. Asian J Pharm Sci 2024;19(4):100939. https://doi.org/10.1016/j.ajps.2024.100939 PMid: 39246507
Ru JL, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 2014;6(1):13. https://doi.org/10.1186/1758-2946-6-13 PMid: 24735618
Liu DY, Zhao HM, Cheng SM, et al. Scorpio and Scolopendra attenuate inflammation and articular damage in rats with collagen-induced arthritis. J Ethnopharmacol 2012;141(2):603-607. https://doi.org/10.1016/j.jep.2011.08.056 PMid: 21911049
Ji LL, Lv SW, Yang ZX. Research progress on chemical constituents and pharmacological effects of centipede. Special Wild Economic Animal and Plant Research 2020;42(04):75-84.
Zhang KY, Zhang YQ, Yang CM, et al. Research progress on the processing history, chemical composition and pharmacological action of Scorpion. China Journal of Chinese Materia Medica 2024;49(04):868-883.
Davis AP, Wiegers TC, Wiegers J, et al. CTD tetramers: a new online tool that computationally links curated chemicals, genes, phenotypes, and diseases to inform molecular mechanisms for environmental health. Toxicol Sci 2023;195(2):155-168. https://doi.org/10.1093/toxsci/kfad069 PMid: 37486259
Otasek D, Morris JH, Bouças J, et al. Cytoscape Automation: Empowering workflow-based network analysis. Genome Biol 2019;20(1):185. https://doi.org/10.1186/s13059-019-1758-4 PMid: 31477170
Szklarczyk D, Kirsch R, Koutrouli M, et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res 2023;51(D1):D638-D646. https://doi.org/10.1093/nar/gkac1000 PMid: 36370105
Griffin RJ, Avery E, Xia CQ. Predicting approximate clinically effective doses in oncology using preclinical efficacy and body surface area conversion: A retrospective analysis. Front Pharmacol 2022;13:830972. https://doi.org/10.3389/fphar.2022.830972 PMid: 35559235
Fan SY, Zhou LJ, Zhang WJ, et al. Ferroptosis: the balance between death and survival in colorectal cancer. Int J Biol Sci 2024;20(10):3773-3783. https://doi.org/10.7150/ijbs.96828 PMid: 39113707
Eng C, Yoshino T, Ruíz-García E, et al. Colorectal cancer. Lancet 2024;404(10449):294-310. https://doi.org/10.1016/S0140-6736(24)00360-X PMid: 38909621
Glover HL, Schreiner A, Dewson G, et al. Mitochondria and cell death. Nat Cell Biol 2024;26(9):1434-1446. https://doi.org/10.1038/s41556-024-01429-4 PMid: 38902422
An XQ, Yu WF, Liu JB, et al. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis 2024;15(8):556. https://doi.org/10.1038/s41419-024-06939-5 PMid: 39090114
Chen AQ, Huang HF, Fang SM, et al. ROS: A “booster” for chronic inflammation and tumor metastasis. Biochim Biophys Acta Rev Cancer 2024;1879(6):189175. https://doi.org/10.1016/j.bbcan.2024.189175 PMid: 39218404
Kang N, Son SB, Min SH, et al. Stimuli-responsive ferroptosis for cancer therapy. Chem Soc Rev 2023;52(12):955-3972. https://doi.org/10.1039/D3CS00001J PMid: 37218295
Qiao Y, Su M, Zhao H, et al. Targeting FTO induces colorectal cancer ferroptotic cell death by decreasing SLC7A11/ GPX4 expression. J Exp Clin Cancer Res 2024;43(1):108. https://doi.org/10.1186/s13046-024-03032-9 PMid: 38600610
Ma X, Cao D, Zhang Y, et al. Apatinib combined with paclitaxel suppresses synergistically TNBC progression through enhancing ferroptosis susceptibility regulated SLC7A11/GPX4/ACSL4 axis. Cell Signal 2025;131:111760. https://doi.org/10.1016/j.cellsig.2025.111760 PMid: 40120963
Liu X, Wang J, Shen K, et al. p53/HIF-1? regulates neuronal aging and autophagy in spinal cord ischemia/reperfusion injury. Mech Ageing Dev 2024;222:112000. https://doi.org/10.1016/j.mad.2024.112000 PMid: 39515667
Wang W, Liu D, Yang L, et al. Compound Kushen injection attenuates angiotensin II?mediated heart failure by inhibiting the PI3K/Akt pathway. Int J Mol Med. 2023;51(3):23. https://doi.org/10.3892/ijmm.2023.5226 PMid: 36734284

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Copyright (c) 2025 Electronic Journal of Biotechnology