Abstract
Background: The protein posttranslational modifications, including ubiquitination and methylation, exhibit the essential function in breast cancer. Herein, we aimed to explore the molecular mechanism of neural precursor cell-expressed developmentally downregulated gene 4-like (NEDD4L) associated with Rho GTPase Rif (RHOF) and AlkB homolog 5 (ALKBH5). A series of experiments including expression detection, cell functions, xenograft tumor assay, and interaction analysis was designed.
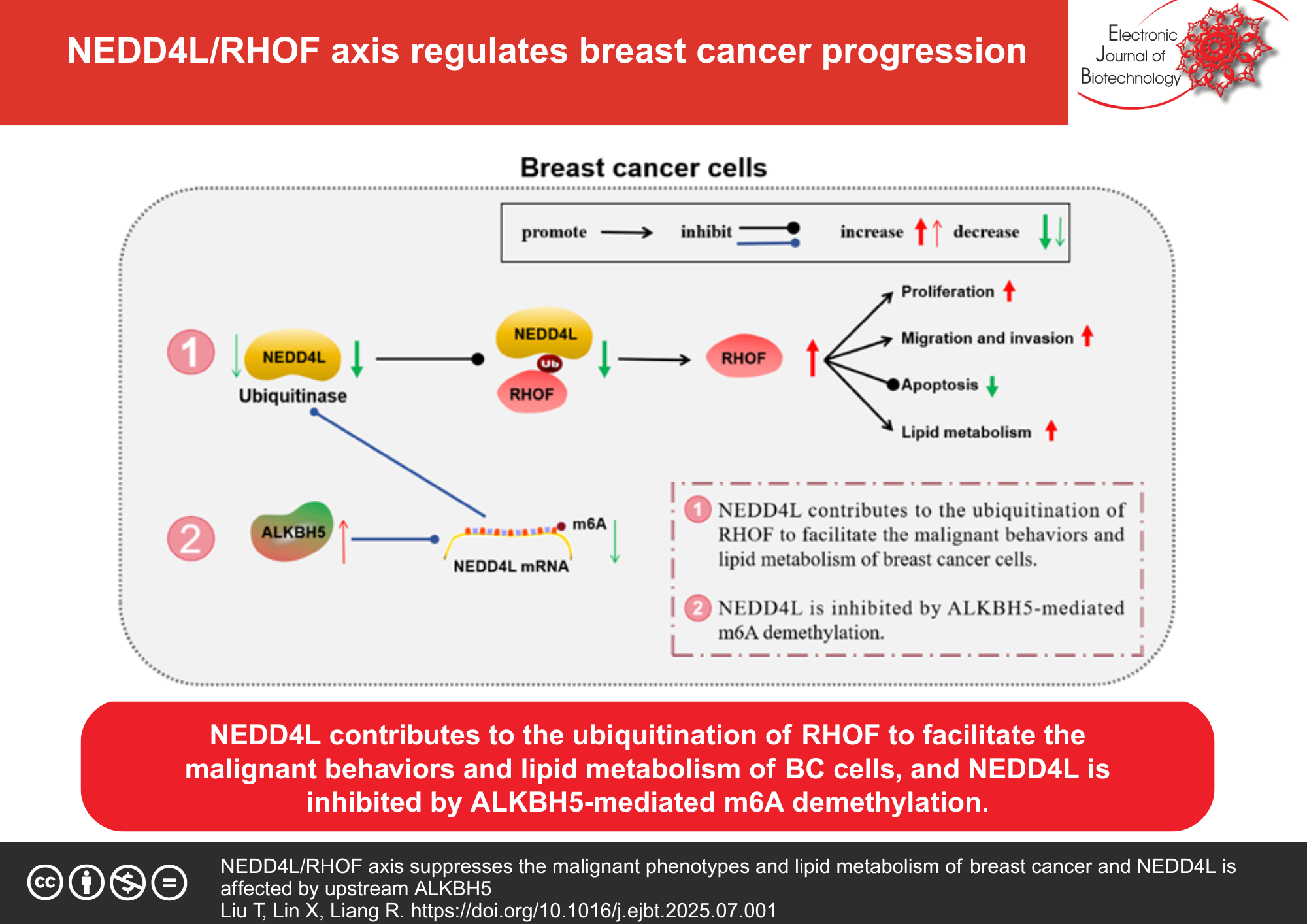
Results: RHOF was up-regulated in breast cancer samples and cells. Silencing RHOF suppressed breast cancer cell growth, migration, invasion and lipid metabolism. Breast cancer tumorigenesis and lipid metabolism were repressed by RHOF knockdown in vivo. NEDD4L impaired RHOF stability by promoting its ubiquitination. NEDD4L overexpression restrained breast cancer cell progression and lipid metabolism via degrading RHOF. ALKBH5 inhibited NEDD4L expression through m6A modification.
Conclusions: These results evidenced that NEDD4L facilitated the malignant progression of breast cancer via inducing the ubiquitination of RHOF, and NEDD4L was also affected by ALKBH5-mediated m6A demethylation.
References
Katsura C, Ogunmwonyi I, Kankam HK, Saha S. Breast cancer: Presentation, investigation and management. Br J Hosp Med 2022;83(2):1-7. https://doi.org/10.12968/hmed.2021.0459 PMid: 35243878
Sadeghi M, Cava C, Moisavi P, et al. Construction of prognostic ceRNA network landscape in breast cancer to explore impacting genes on drug response by integrative bioinformatics analysis. Lett Drug Design Discov 2024;21(12):2467-2481. https://doi.org/10.2174/0115701808255183230922110002
Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263. https://doi.org/10.3322/caac.21834 PMid: 38572751
Rossi L, Mazzara C, Pagani O. Diagnosis and treatment of breast cancer in young women. Curr Treat Options Oncol. 2019;20(12):86. https://doi.org/10.1007/s11864-019-0685-7 PMid: 31776799
Loibl S, Poortmans P, Morrow M, et al. Breast cancer. The Lancet 2021;397(10286):1750-1769. https://doi.org/10.1016/S0140-6736(20)32381-3 PMid: 33812473
Xiong X, Zheng LW, Ding Y, et al. Breast cancer: Pathogenesis and treatments. Signal Transduct Target Ther. 2025;10(1):49. https://doi.org/10.1038/s41392-024-02108-4 PMid: 39966355
Rusnáková DS, Aziri R, Dubovan P, et al. Detection, significance and potential utility of circulating tumor cells in clinical practice in breast cancer. Oncol Lett. 2025;29(1):10. https://doi.org/10.3892/ol.2024.14756 PMid: 39492933
Mosaddeghzadeh N, Ahmadian MR. The RHO family GTPases: Mechanisms of regulation and signaling. Cells. 2021;10(7):1831. https://doi.org/10.3390/cells10071831 PMid: 34359999
Crosas-Molist E, Samain R, Kohlhammer L, et al. RHO GTPase signaling in cancer progression and dissemination. Physiol Rev. 2022;102(1):455-510. https://doi.org/10.1152/physrev.00045.2020 PMid: 34541899
Zhao R, Yi Y, Liu H, et al. RHOF promotes Snail1 lactylation by enhancing PKM2-mediated glycolysis to induce pancreatic cancer cell endothelial-mesenchymal transition. Cancer Metab. 2024;12(1):32. https://doi.org/10.1186/s40170-024-00362-2 PMid: 39462429
Yang RM, Zhan M, Xu SW, et al. miR-3656 expression enhances the chemosensitivity of pancreatic cancer to gemcitabine through modulation of the RHOF/EMT axis. Cell Death Dis. 2017;8(10):e3129. https://doi.org/10.1038/cddis.2017.530 PMid: 29048402
Wen X, Li P, Ma Y, et al. RHOF activation of AKT/?-catenin signaling pathway drives acute myeloid leukemia progression and chemotherapy resistance. iScience. 2024;27(7):110221. https://doi.org/10.1016/j.isci.2024.110221 PMid: 39021805
Luo J, Chen S, Chen J, et al. Identification and validation of DNA methylation markers to predict axillary lymph node metastasis of breast cancer. PLoS ONE. 2022;17(12):e0278270. https://doi.org/10.1371/journal.pone.0278270 PMid: 36454866
Zhang M, Zhang Z, Tian X, et al. NEDD4L in human tumors: Regulatory mechanisms and dual effects on anti-tumor and pro-tumor. Front Pharmacol. 2023;14:1291773. https://doi.org/10.3389/fphar.2023.1291773 PMid: 38027016
Dong H, Zhu L, Sun J, et al. Pan-cancer analysis of NEDD4L and its tumor suppressor effects in clear cell renal cell carcinoma. J Cancer. 2021;12(20):6242-6253. https://doi.org/10.7150/jca.58004 PMid: 34539897
Shi Y, Fang N, Wu Y, et al. NEDD4L mediates ITGB4 ubiquitination and degradation to suppress esophageal carcinoma progression. Cell Commun Signal. 2024;22(1):302. https://doi.org/10.1186/s12964-024-01685-9 PMid: 38831335
Guarnieri AL, Towers CG, Drasin DJ, et al. The miR-106b-25 cluster mediates breast tumor initiation through activation of NOTCH1 via direct repression of NEDD4l. Oncogene. 2018;37(28):3879-3893. https://doi.org/10.1038/s41388-018-0239-7 PMid: 29662198
Liu L, Zhang C, Qu S, et al. ESR1 inhibits ionizing radiation-induced ferroptosis in breast cancer cells via the NEDD4L/CD71 pathway. Arch Biochem Biophys. 2022;725:109299. https://doi.org/10.1016/j.abb.2022.109299 PMid: 35613689
Zhang G, Cheng C, Wang X, et al. N6-Methyladenosine methylation modification in breast cancer: Current insights. J Transl Med. 2024;22(1):971. https://doi.org/10.1186/s12967-024-05771-x PMid: 39468547
Han X, Ren C, Jiang A, et al. Arginine methylation of ALKBH5 by PRMT6 promotes breast tumorigenesis via LDHA-mediated glycolysis. Front Med. 2024;18(2):344-356. https://doi.org/10.1007/s11684-023-1028-4 PMid: 38466502
Lyu Y, Wang Y, Ding H, et al. Hypoxia-induced m6A demethylase ALKBH5 promotes ovarian cancer tumorigenicity by decreasing methylation of the lncRNA RMRP. Am J Cancer Res. 2023;13(9):4179-4191. PMid: 37818080
Bian X, Liu R, Meng Y, et al. Lipid metabolism and cancer. J Exp Med. 2021;218(1):e20201606. https://doi.org/10.1084/jem.20201606 PMid: 33601415
Chen J, Ying K, Sun J, et al. NEDD4l affects KLF5 stability through ubiquitination to control ferroptosis and radiotherapy resistance in oesophageal squamous cell carcinoma. J Cell Mol Med. 2024;28(18):e70062. https://doi.org/10.1111/jcmm.70062 PMid: 39317954
Kim YH, Yoo H, Hong AR, et al. NEDD4L limits camp signaling through ubiquitination of CREB-regulated transcription coactivator 3. FASEB J. 2018;32(7):4053-4062. https://doi.org/10.1096/fj.201701406R PMid: 29505301
Li Z, Wei H, Li S, et al. The role of progesterone receptors in breast cancer. Drug Des Devel Ther. 2022;16:305-314. https://doi.org/10.2147/DDDT.S336643 PMid: 35115765
Zhao H, Liang Y, Sun C, et al. Dihydrotanshinone I inhibits the lung metastasis of breast cancer by suppressing neutrophil extracellular traps formation. Int J Mol Sci. 2022;23(23):1510. https://doi.org/10.3390/ijms232315180 PMid: 36499502
Zeng L, Zhou G, Yang W, et al. Guidelines for the diagnosis and treatment of knee osteoarthritis with integrative medicine based on traditional Chinese medicine. Front Med 2023;10:1260943. https://doi.org/10.3389/fmed.2023.1260943 PMid: 37915321
Aranda-Rivera AK, Cruz-Gregorio A, Aparicio-Trejo OE, et al. Sulforaphane protects against unilateral ureteral obstruction-induced renal damage in rats by alleviating mitochondrial and lipid metabolism impairment. Antioxidants 2022;11(10):1854. https://doi.org/10.3390/antiox11101854 PMid: 36290577
Shaverdashvili K, Padlo J, Weinblatt D, et al. KLF4 activates NF?B signaling and esophageal epithelial inflammation via the Rho-related GTP-binding protein RHOF. PLoS ONE 2019;14(4):e0215746. https://doi.org/10.1371/journal.pone.0215746 PMid: 30998758
Yin X, Xu R, Song J, et al. Lipid metabolism in pancreatic cancer: Emerging roles and potential targets. Cancer Commun 2022;42(12):1234-1256. https://doi.org/10.1002/cac2.12360 PMid: 36107801
Broadfield LA, Pane AA, Talebi A, et al. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev Cell. 2021;56(10):1363-1393. https://doi.org/10.1016/j.devcel.2021.04.013 PMid: 33945792
Corn KC, Windham MA, Rafat M. Lipids in the tumor microenvironment: From cancer progression to treatment. Prog Lipid Res. 2020;80:101055. https://doi.org/10.1016/j.plipres.2020.101055 PMid: 32791170
Xiang Y, Miao H. Lipid metabolism in tumor-associated macrophages. In: Li Y (editor) Lipid metabolism in tumor immunity. Advances in Experimental Medicine and Biology, Springer, Singapore. 2021;1316:87-101. https://doi.org/10.1007/978-981-33-6785-2_6 PMid: 33740245
Zhang R, Chen J, Wang S, et al. Ferroptosis in cancer progression. Cells. 2023;12(14):1820. https://doi.org/10.3390/cells12141820 PMid: 37508485
Fhu CW, Ali A. Fatty acid synthase: An emerging target in cancer. Molecules. 2020;25(17):3935. https://doi.org/10.3390/molecules25173935 PMid: 32872164
Menendez JA, Lupu R. Fatty acid synthase: A druggable driver of breast cancer brain metastasis. Expert Opin Ther Targets. 2022;26(5):427-444. https://doi.org/10.1080/14728222.2022.2077189 PMid: 35545806
He Y, Qi S, Chen L, et al. The roles and mechanisms of SREBP1 in cancer development and drug response. Genes Dis. 2024;11(4):100987. https://doi.org/10.1016/j.gendis.2023.04.022 PMid: 38560498
Liu F, Chen J, Li K, et al. Ubiquitination and deubiquitination in cancer: From mechanisms to novel therapeutic approaches. Mol Cancer. 2024;23(1):148. https://doi.org/10.1186/s12943-024-02046-3 PMid: 39048965
Dewson G, Eichhorn PJA, Komander D. Deubiquitinases in cancer. Nat Rev Cancer. 2023;23(12):842-862. https://doi.org/10.1038/s41568-023-00633-y PMid: 37935888
Guo Y, Cui Y, Li Y, et al. Cytoplasmic YAP1-mediated ESCRT-III assembly promotes autophagic cell death and is ubiquitinated by NEDD4L in breast cancer. Cancer Commun 2023;43(5):582-612. https://doi.org/10.1002/cac2.12417 PMid: 37005481
Singh S, Gupta S, Abhishek R, et al. Regulation of m6A (N6-Methyladenosine) methylation modifiers in solid cancers. Funct Integr Genomics. 2024;24(6):193. https://doi.org/10.1007/s10142-024-01467-z PMid: 39438339
Hu Y, Gong C, Li Z, et al. Demethylase ALKBH5 suppresses invasion of gastric cancer via PKMYT1 m6a modification. Mol Cancer. 2022;21(1):34. https://doi.org/10.1186/s12943-022-01522-y PMid: 35114989
Zhai J, Chen H, Wong CC, et al. ALKBH5 drives immune suppression via targeting AXIN2 to promote colorectal cancer and is a target for boosting immunotherapy. Gastroenterology. 2023;165(2):445-462. https://doi.org/10.1053/j.gastro.2023.04.032 PMid: 37169182

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Copyright (c) 2025 Electronic Journal of Biotechnology