Abstract
Background: Understanding the dynamics of fungal communities in composting and vermicomposting systems is essential for optimizing waste management practices and minimizing pathogen risks. For this reason, this study assessed the fungal community structure and potential pathogenic risks in composting, vermicomposting and leachate systems amended with rabbit manure, using Illumina’s MiSeq platform for internal transcribed spacer (ITS) sequencing and FUNGuild analysis.
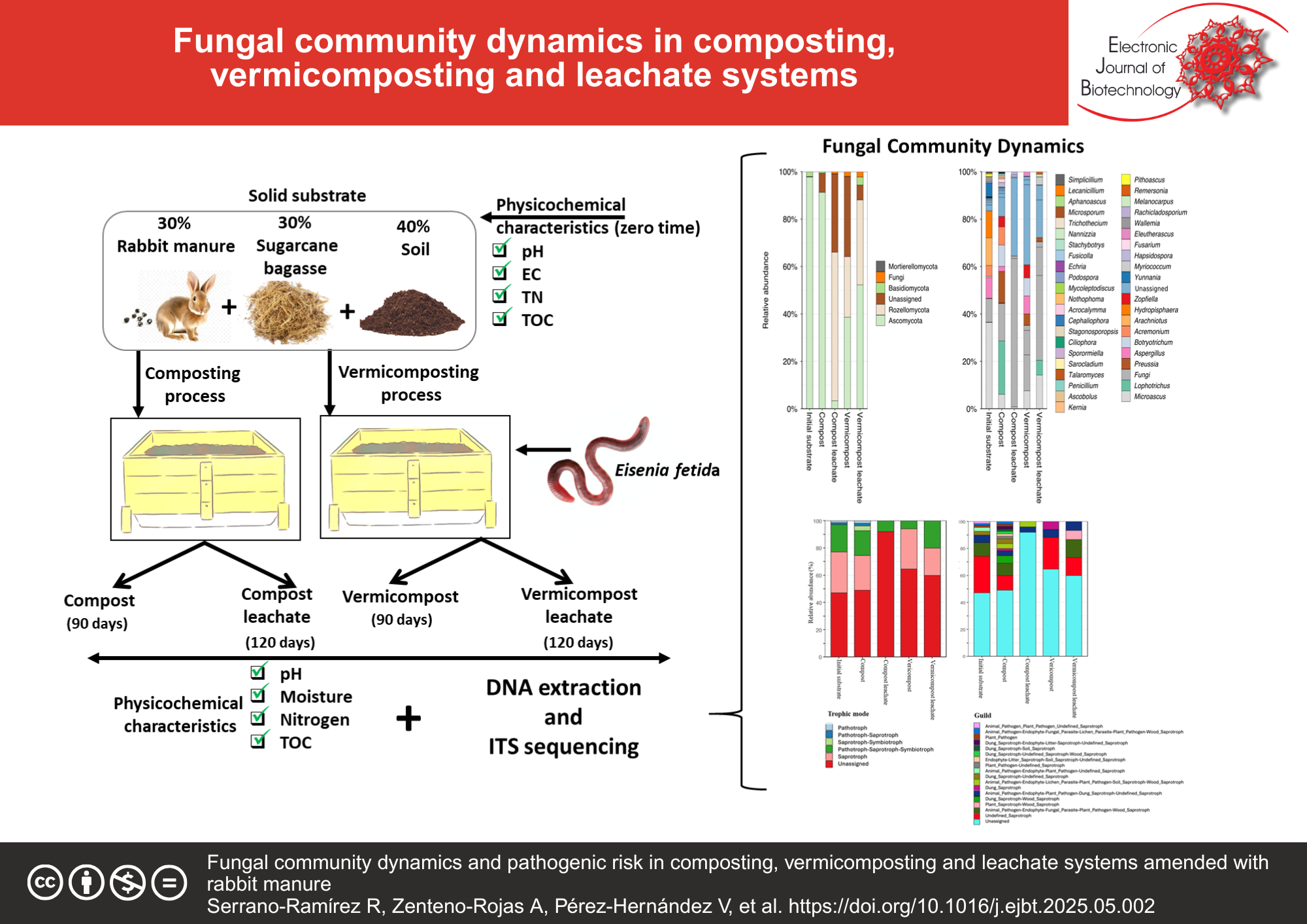
Results: Phylotypes from Basidiomycota were predominant in all treatments, while the pathogenic genus Microascus, initially abundant (37%), showed a significant reduction to 5% and 7% following composting and vermicomposting, respectively, and to 1% and 13% in their leachates. Given Microascus’s association with human skin diseases, proper handling of organic waste is critical before its agricultural use. In contrast, the FUNGuild analysis revealed a high abundance of saprotrophic fungi such as Aspergillus, Preussia, Botryotrichum, and Acremonium known for producing enzymes that promote nutrient cycling and soil fertility.
Conclusions: These findings highlight the potential for vermicomposting to reduce pathogen risks while enhancing fungal-driven nutrient recycling, offering practical insights for sustainable agriculture and organic waste management.
References
Sayara T, Basheer-Salimia R, Hawamde F, et al. Recycling of organic wastes through composting: Process performance and compost application in agriculture. Agronomy 2020;10(11):1838. https://doi.org/10.3390/agronomy10111838
Lin C, Cheruiyot NK, Bui XT, et al. Composting and its application in bioremediation of organic contaminants. Bioengineered 2022;13(1):1073–1089. https://doi.org/10.1080/21655979.2021.2017624 PMid: 35001798
Vázquez-Villegas PT, Meza-Gordillo R, Cruz-Salomón A, et al. Vermicomposting process to endosulfan lactone removal in solid substrate using Eisenia fetida. Processes 2021;9(2):396. https://doi.org/10.3390/pr9020396
Bouhia Y, Hafidi M, Ouhdouch Y, et al. Olive mill waste sludge: From permanent pollution to a highly beneficial organic biofertilizer: A critical review and future perspectives. Ecotoxicol Environ Saf 2023;259:114997. https://doi.org/10.1016/j.ecoenv.2023.114997 PMid: 37210993
Pampillón-González L, Ortiz-Cornejo NL, Luna-Guido M, et al. Archaeal and bacterial community structure in an anaerobic digestion reactor (Lagoon Type) used for biogas production at a pig farm. J Mol Microbiol 2017;27(5):306-317. https://doi.org/10.1159/000479108 PMid: 29186720
Gutiérrez-Miceli FA, García-Gómez RC, Oliva-Llaven MA, et al. Vermicomposting leachate as liquid fertilizer for the cultivation of sugarcane (Saccharum sp.). J Plant Nutr 2016;40(1):40-49. https://doi.org/10.1080/01904167.2016.1193610
Sall PM, Antoun H, Chalifour F, et al. Potential use of leachate from composted fruit and vegetable waste as fertilizer for corn and vegetable waste as fertilizer for corn. Cogent Food Agric 2019;5(1):1580180. https://doi.org/10.1080/23311932.2019.1580180
Hernández-Lara A, Ros M, Cuartero J, et al. Bacterial and fungal community dynamics during different stages of agro-industrial waste composting and its relationship with compost suppressiveness. Sci Total Environ 2022;805:150330. https://doi.org/10.1016/j.scitotenv.2021.150330 PMid: 34818753
Fierer N. Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol 2017;15(10):579-590. https://doi.org/10.1038/nrmicro.2017.87 PMid: 28824177
Anastasi A, Varese GC, Voyron S, et al. Characterization of fungal biodiversity in compost and vermicompost. Compost Sci Util 2013;12(4):185-191. https://doi.org/10.1080/1065657X.2004.10702179
Liggenstoffer AS, Youssef NH, Couger MB, et al. Phylogenetic diversity and community structure of anaerobic gut fungi (phylum Neocallimastigomycota) in ruminant and non-ruminant herbivores. The ISME Journal 2010;4(10):1225-1235. https://doi.org/10.1038/ismej.2010.49 PMid: 20410935
Pavan HV, Murthy SM, Jogaiah S. Explorations of fungal diversity in extreme environmental conditions for sustainable agriculture applications. In: Jogaiah S editor, Biocontrol Agents and Secondary Metabolites. Woodhead Publishing, 2021. pp. 483-494. https://doi.org/10.1016/b978-0-12-822919-4.00021-1
Gloor, G.B., Fernandes, A.D., Macklaim, J.M. Analysis of differential abundance taking sample variation into account: Package “ALDEx2”. 2020. [cited April 12, 2024]. Available at: https://github.com/ggloor/ALDEx_bioc
Okuda Y. Sustainability perspectives for future continuity of mushroom production: The bright and dark sides. Front Sustain Food Syst 2022;6:1026508 https://doi.org/10.3389/fsufs.2022.1026508
Vyas P, Sharma S, Gupta J. Vermicomposting with microbial amendment: implications for bioremediation of industrial and agricultural waste. BioTechnologia 2022;103(2):203-215. https://doi.org/10.5114/bta.2022.116213
Serrano-Ramírez RP, Hernández-Guzmán M, Ruiz-Valdiviezo VM, et al. Study of bacterial communities in different types of leachates and their impact on Solanum lycopersicum production in greenhouses. J Soil Sci Plant Nutr 2021;21:1170-1181. https://doi.org/10.1007/s42729-021-00430-2
Zhong XZ, Li XX, Zeng Y, et al. Dynamic change of bacterial community during dairy manure composting process revealed by high-throughput sequencing and advanced bioinformatics tools. Bioresour Technol 2020;306:123091. https://doi.org/10.1016/j.biortech.2020.123091
Romero-Tepal EM, Contreras-Blancas E, Navarro-Noya YE, et al. Changes in the bacterial community structure in stored wormbed leachate. J Mol Microbiol 2014;24(2):105-113. https://doi.org/10.1159/000357915
Association of Official Analytical Chemists (AOAC) International. 2000. Official Methods of Analysis (17th ed., Vol. II). Gaithersburg, Maryland, USA: AOAC International.
Hoffman CS, Winston FA. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2-3):267-272. https://doi.org/10.1016/0378-1119(87)90131-4
Sambrook, J., Russell, D.W. Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press. 2001. [cited January 27, 2024]. Available at: https://www.cshlpress.com/pdf/sample/2013/MC4/MC4FM.pdf
Valenzuela-Encinas C, Neria-González I, Alcántara-Hernández RJ, et al. Phylogenetic analysis of the archaeal community in an alkaline-saline soil of the former lake Texcoco (Mexico). Extremophiles 2008;12(2):247-254. https://doi.org/10.1007/s00792-007-0121
Montoya-Ciriaco N, Gómez-Acata S, Muñoz-Arenas LC, et al. Dietary effects on gut microbiota of the mesquite lizard Sceloporus grammicus (Wiegmann, 1828) across different altitudes. Microbiome 2020;8(1):6. https://doi.org/10.1186/s40168-020-0783-6
White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. editors. PCR protocols: A guide to methods and applications, Academic Press. 1990:315-322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Andrews, S. FastQC: A quality control tool for high throughput sequence data. Babraham Institute. 2019. [cited September 2, 2023]. Available at: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019;37:852-857. https://doi.org/10.1038/s41587-019-0209-9
Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 2016;13:581-583. https://doi.org/10.1038/nmeth.3869
Kõljalg U, Nilsson RH, Abarenkov K, et al. Towards a unified paradigm for sequence-based identification of fungi. Molecular Ecology 20213;22(21):5271-5277. https://doi.org/10.1111/mec.12481
Nguyen NH, Song Z, Bates ST, et al. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecology 2016;20:241-248. https://doi.org/10.1016/J.FUNECO.2015.06.006
R Core Team. R: A Language and Environment for Statistical Computing. 2020. [cited April 12, 2024]. Available at: https://www.r-project.org
Bisanz, J.E., 2018. qiime2R: Importing QIIME2 artifacts and associated data into R sessions. 2018. [cited May 28, 2024]. Available at: https://github.com/jbisanz/qiime2R
McMurdie PJ, Holmes S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013;8(4):e61217. https://doi.org/10.1371/journal.pone.0061217
Wickham H. ggplot2: Elegant graphics for data analysis. Springer-Verlag. New York. 2016. https://ggplot2.tidyverse.org
Oksanen, J., Blanchet, F.G., Friendly, M., et al. Wagner, H. Vegan: Community ecology package. (version 2.5) 2019. [cited December 22, 2023]. Available at: https://CRAN.R-project.org/package=vegan
Quinn TP, Erb I, Gloor G, et al. A field guide for the compositional analysis of any-omics data. GigaScience. 2019;8(9):giz107. https://doi.org/10.1093/gigascience/giz107
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;5(12):550. https://doi.org/10.1186/s13059-014-0550-8
Conway JR, Lex A, Gehlenborg N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017;33(18):2938-2940. https://doi.org/10.1093/bioinformatics/btx364
Landínez-Torres AY, Becerra-Abril JL, Tosi S, et al. Soil microfungi of the Colombian natural regions. Int J Environ Res Public Health 2020;17(22):8311. https://doi.org/10.3390/ijerph17228311
Liu B, Wu C, Pan P, et al. Remediation effectiveness of vermicompost for a potentially toxic metal contaminated tropical acidic soil in China. Ecotox Environ Safe 2019;182:109394. https://doi.org/10.1016/j.ecoenv.2019.109394
Pan P, Liu H, Liu A, et al. Rhizosphere environmental factors regulated the cadmium adsorption by vermicompost: Influence of pH and low-molecular-weight organic acids. Ecotox Environ Safe 2023;266:115593. https://doi.org/10.1016/j.ecoenv.2023.115593
Wang F, Zhang W, Miao L, et al. The effects of vermicompost and shell powder addition on Cd bioavailability, enzyme activity and bacterial community in Cd-contaminated soil: A field study. Ecotox Environ Safe 2021;215:112163. https://doi.org/10.1016/j.ecoenv.2021.112163
Ravindran C, Naveenan T. Adaptation of marine derived fungus Chaetomium globosum (NIOCC 36) to alkaline stress using antioxidant properties. Process Biochem 2011;46(4):847-857. https://doi.org/10.1016/j.procbio.2010.12.005
Ramnarain YI, Ansari AA, Ori L. Vermicomposting of different organic materials using the epigeic earthworm Eisenia foetida. IJROWA 2019;8(1):23-36. https://doi.org/10.1007/s40093-018-0225-7
Ordoñez-Arévalo B, Guillén-Navarro K, Huerta E, et al. Enzymatic dynamics into the Eisenia fetida (Savigny, 1826) gut during vermicomposting of coffee husk and market waste in a tropical environment. Environ Sci Pollut Res 2018;25(2):1576-1586. https://doi.org/10.1007/s11356-017-0572-3
Suthar S. Nutrient changes and bio dynamics of epigeic earthworm Perionyx excavatus (Perrier) during recycling of some agriculture wastes. Bioresour Technol 2007;98(8):1608-1614. https://doi.org/10.1016/j.biortech.2006.06.001
Usmani Z, Kumar V, Rani R, et al. Changes in physico-chemical, microbiological and biochemical parameters during composting and vermicomposting of coal fly ash: A comparative study. Int J Environ Sci Technol 2019;16(8):4647-4664. https://doi.org/10.1007/s13762-018-1893-6
Yuvaraj A, Thangaraj R, Ravindran B, et al. Centrality of cattle solid wastes in vermicomposting technology - A cleaner resource recovery and biowaste recycling option for agricultural and environmental sustainability. Environ Pollut 2021;268(PtA):115688. https://doi.org/10.1016/j.envpol.2020.115688
Gutiérrez-Miceli FA, Oliva-Llaven MA, Mendoza-Nazar P, et al. Optimization of vermicompost and worm-bed leachate for the organic cultivation of radish. J Plant Nutr 2011;34(11):1642-1653.
El-Gindy AA. Production of cellulase(s) by Myriococcum albomyces. Zentralblatt für Mikrobiologie 1991;146(3):193-196. https://doi.org/10.1016/S0232-4393(11)80180-0
Seddouk L, Jamai L, Tazi K, et al. Isolation and characterization of a mesophilic cellulolytic endophyte Preussia africana from Juniperus oxycedrus. Environ Sci Pollut Res 2022;29(30):45589-45600. https://doi.org/10.1007/s11356-022-19151-9
Garg VK, Kaushik P. Vermistabilization of textile mill sludge spiked with poultry droppings by an epigeic earthworm Eisenia foetida. Bioresour Technol 2005;96(9):1063-1071. https://doi.org/10.1016/j.biortech.2004.09.003
Suthar S. Potential utilization of guar gum industrial waste in vermicompost production. Bioresour Technol 2006;97(18):2474-2477. https://doi.org/10.1016/j.biortech.2005.10.018
Neher DA, Weicht TR, Bates ST, et al. Changes in bacterial and fungal communities across compost recipes, preparation methods, and composting times. PLoS ONE 2013;8(11):e79512. https://doi.org/10.1371/journal.pone.0079512
Meng Q, Yang W, Men M, et al. Microbial community succession and response to environmental variables during cow manure and corn straw composting. Front Microbiol 2016;10:529. https://doi.org/10.3389/fmicb.2019.00529
Irawan B, Saputra A, Farisi S, et al. The use of cellulolytic Aspergillus sp. inoculum to improve the quality of Pineapple compost. AIMS Microbiol 2023;9(1):41-54. https://doi.org/10.3934/microbiol.2023003
Sun X, Zhang C, Bei S, et al. High bacterial diversity and siderophore-producing bacteria collectively suppress Fusarium oxysporum in maize/faba bean intercropping. Front Microbiol 2022;13:972587. https://doi.org/10.3389/fmicb.2022.972587
Dong M, Shao Y, Xu Z, et al. Resilience of fungal flora in bauxite residues amended with organic matter and vermiculite/fly ash. J Environ Manage 2021;284:112052. https://doi.org/10.1016/j.jenvman.2021.112052
Frey SD, Knorr M, Parrent JL, et al. Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. Forest Ecol Manag 2004;196(1):159–171. https://doi.org/10.1016/j.foreco.2004.03.018
Jaber LR, Vidal S. Interactions between an endophytic fungus, aphids, and extrafloral nectaries: Do endophytes induce extrafloral-mediated defences in Vicia faba? Funct Ecol 2009;23(4):707-714. https://doi.org/10.1111/j.1365-2435.2009.01554.x
Kasselaki AM, Shaw MW, Malathrakis NE, et al. Control of Leveillula taurica in tomato by Acremonium alternatum is by induction of resistance, not hyperparasitism. Eur J Plant Pathol 2006;115(2):263-267. https://doi.org/10.1007/s10658-006-9010-y
Shang SQ, Chen YN, Bai YL. The pathogenicity of entomopathogenic fungus Acremonium hansfordii to two-spotted spider mite, Tetranychus urticae and predatory mite Neoseiulus barkeri. Syst Appl Acarol 2018;23(11):2173-2183. https://doi.org/10.11158/saa.23.11.10
Yang W, Jing X, Guan Y, et al. Response of fungal communities and co-occurrence network patterns to compost amendment in black soil of Northeast China. Front Microbiol 2019;10:1562. https://doi.org/10.3389/fmicb.2019.01562
Moreno J, López-González JA, Arcos-Nievas MA, et al. Revisiting the succession of microbial populations throughout composting: A matter of thermotolerance. Sci Total Environ 2021;773:145587. https://doi.org/10.1016/j.scitotenv.2021.145587
Amobonye A, Aruwa CE, Aransiola S, et al. The potential of fungi in the bioremediation of pharmaceutically active compounds: A comprehensive review. Front Microbiol 2023;14:1207792. https://doi.org/10.3389/fmicb.2023.1207792
Frac M, Hannula SE, Be?ka M, et al. Fungal biodiversity and their role in soil health. Front Microbiol 2018;9:707. https://doi.org/10.3389/fmicb.2018.00707
Ning JY, Zhu XD, Zu HG, et al. Coupling thermophilic composting and vermicomposting processes to remove Cr from biogas residues and produce high value-added biofertilizers. Bioresour Technol 2021;329:124869. https://doi.org/10.1016/j.biortech.2021.124869
Ayilara MS, Olanrewaju OS, Babalola OO, et al. Waste management through composting: Challenges and potentials. Sustainability 2021;12(11):4456. https://doi.org/10.3390/su12114456
De Corato U, Patruno L, Avella N, et al. Composts from green sources show an increased suppressiveness to soilborne plant pathogenic fungi: Relationships between physicochemical properties, disease suppression, and the microbiome. Crop Prot 2019;124:104870. https://doi.org/10.1016/j.cropro.2019.104870
Lazcano C, Gómez-Brandón M, Domínguez J. Comparison of the effectiveness of composting and vermicomposting for the biological stabilization of cattle manure. Chemosphere 2008;72(7):1013-1019. https://doi.org/10.1016/j.chemosphere.2008.04.016
Ducasse V, Capowiez Y, Peigné J. Vermicomposting of municipal solid waste as a possible lever for the development of sustainable agriculture. A review. Agron Sustain Dev 2022;42:89. https://doi.org/10.1007/s13593-022-00819-y

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Copyright (c) 2025 Electronic Journal of Biotechnology