Abstract
Background: The rise of drug-resistant bacteria, including Staphylococcus aureus and Escherichia coli, presents a significant healthcare challenge. This study focuses on the development of two novel nanocomposites IONs-MWCNTs-Pc and IONs-GO-Pc encapsulated within a biocompatible poly(VCL-co-PEGDA) hydrogel. These composites are designed for use in photodynamic therapy and evaluated for their antimicrobial efficacy against resistant pathogens.
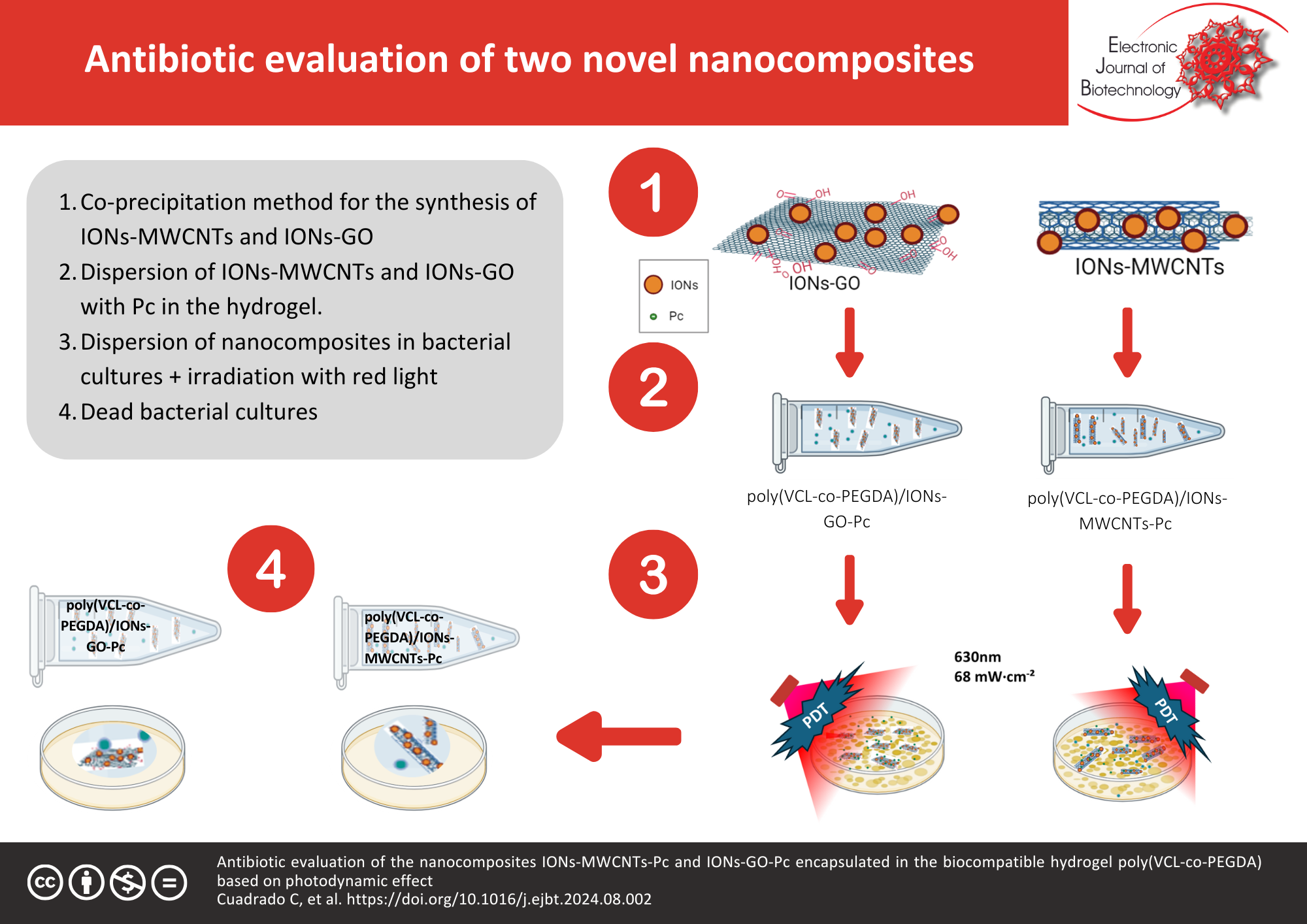
Results: The synthesized nanocomposites, when irradiated with red light at 630 nm, showed significant antimicrobial activity, resulting in a marked reduction in the viability of S. aureus ATCC 27543, S. aureus ATCC 33591, and E. coli ATCC 971182. Photodynamic studies demonstrated that the IONs-GO-Pc nanocomposite was more efficient in generating singlet oxygen compared to IONs-MWCNTs-Pc, which correlated with its superior antimicrobial performance. Structural and chemical characterization confirmed the successful incorporation of nanomaterials and photosensitizers, enhancing the photodynamic effect.
Conclusions: The study demonstrates that both IONs-MWCNTs-Pc and IONs-GO-Pc nanocomposites show promise as alternative treatments for infections caused by antibiotic-resistant bacteria, with the GO-based composite showing higher photodynamic therapy efficacy. These findings suggest that such nanocomposites could play a pivotal role in advancing antimicrobial strategies against resistant pathogens.
References
World Health Organization. Managing epidemics: key facts about major deadly diseases. 1st ed. Vol. 1, World Health Organization. Luxembourg: World Health Organization; 2018.
Makabenta JMV, Nabawy A, Li C-H, et al. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol 2021;19(1):23–36. https://doi.org/10.1038/s41579-020-0420-1 PMid: 32814862
Dadgostar P. Antimicrobial resistance: Implications and costs. Infect Drug Resist 2019;12:3903–10. https://doi.org/10.2147/IDR.S234610 PMid: 31908502
World Health Organization. Antimicrobial resistance. 2023 [cited 2023 Apr 6]. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
Ali A, Ovais M, Cui X, et al. Safety assessment of nanomaterials for antimicrobial applications. Chem Res Toxicol 2020;33(5):1082–109. https://doi.org/10.1021/acs.chemrestox.9b00519 PMid: 32302095
Zhao Y, Zhang Z, Pan Z, et al. Advanced bioactive nanomaterials for biomedical applications. Exploration 2021;1(3):20210089. https://doi.org/10.1002/EXP.20210089
Zhu H, Cheng P, Chen P, et al. Recent progress in the development of near-infrared organic photothermal and photodynamic nanotherapeutics. Biomater Sci 2018;6(4):746–65. https://doi.org/10.1039/C7BM01210A PMid:29485662
Wang Y, Wei T, Qu Y, et al. Smart, photothermally activated, antibacterial surfaces with thermally triggered bacteria-releasing properties. ACS Appl Mater Interfaces 2020;12(19):21283–91. https://doi.org/10.1021/acsami.9b17581 PMid:31709795
Menezes BRC de, Rodrigues KF, Fonseca BC da S, et al. Recent advances in the use of carbon nanotubes as smart biomaterials. J Mater Chem B 2019;7(9):1343–60. https://doi.org/10.1039/C8TB02419G PMid: 32255006
Wang C, Makvandi P, Zare EN, et al. Advances in antimicrobial organic and inorganic nanocompounds in biomedicine. Adv Ther 2020;3(8):2000024. https://doi.org/10.1002/adtp.202000024
Al-Jumaili A, Alancherry S, Bazaka K, et al. Review on the antimicrobial properties of carbon nanostructures. Materials 2017;10(9):1066. https://doi.org/10.3390/ma10091066
Klausen M, Ucuncu M, Bradley M. Design of photosensitizing agents for targeted antimicrobial photodynamic therapy. Molecules 2020;25(22):5239. https://doi.org/10.3390/molecules25225239 PMid: 33182751
VT A, Paramanantham P, SB S, et al. Antimicrobial photodynamic activity of rose bengal conjugated multi walled carbon nanotubes against planktonic cells and biofilm of Escherichia coli. Photodiagnosis Photodyn Ther 2018;24:300–10. https://doi.org/10.1016/j.pdpdt.2018.10.013 PMid: 30342101
Wu W, Wu Z, Yu T, et al. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater 2015;16(2):023501. https://doi.org/10.1088/1468-6996/16/2/023501 PMid: 27877761
de Toledo L de AS, Rosseto HC, Bruschi ML. Iron oxide magnetic nanoparticles as antimicrobials for therapeutics. Pharm Dev Technol 2018;23(4):316–23. https://doi.org/10.1080/10837450.2017.1337793 PMid: 28565928
Berry CC, Curtis ASG. Functionalisation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys 2003;36(13):R198–206. https://doi.org/10.1088/0022-3727/36/13/203
Pankhurst QA, Connolly J, Jones SK, et al. Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 2003;36(13):R167–81. https://doi.org/10.1088/0022-3727/36/13/201
Colombo M, Carregal-Romero S, Casula MF, et al. Biological applications of magnetic nanoparticles. Chem Soc Rev 2012;41(11):4306-34. https://doi.org/10.1039/c2cs15337h PMid: 22481569
Cheng L, Wang C, Feng L, et al. Functional nanomaterials for phototherapies of cancer. Chem Rev 2014;114(21):10869–939. https://doi.org/10.1021/cr400532z PMid: 25260098
Kausar A. Nanocarbon in polymeric nanocomposite hydrogel—design and multi-functional tendencies. Polymer-Plastics Technology and Materials 2020;59(14):1505–21. https://doi.org/10.1080/25740881.2020.1757106
Yi J, Choe G, Park J, Lee JY. Graphene oxide-incorporated hydrogels for biomedical applications. Polym J 2020;52(8):823–37. https://doi.org/10.1038/s41428-020-0350-9
Teradal NL, Jelinek R. Carbon nanomaterials in biological studies and biomedicine. Adv Healthc Mater 2017;6(17):1700574. https://doi.org/10.1002/ADHM.201700574 PMid: 28777502
Maiti D, Tong X, Mou X, et al. Carbon-based nanomaterials for biomedical applications: A recent study. Front Pharmacol 2019;9:1401. https://doi.org/10.3389/fphar.2018.01401 PMid: 30914959
Patil TV, Patel DK, Dutta SD, et al. Carbon nanotubes-based hydrogels for bacterial eradiation and wound-healing applications. Applied Sciences 2021;11(20):9550. https://doi.org/10.3390/app11209550
Romero JF, Díaz-Barrios A, González G. Biocompatible thermo-responsive N-vinylcaprolactam based hydrogels for controlled drug delivery systems. Bionatura 2021;6(2):1712–9. https://doi.org/10.21931/RB/2021.06.02.8
Soler MAG, Qu F. Raman spectroscopy of iron oxide nanoparticles. In: Kumar CSSR, editor, Raman Spectroscopy for Nanomaterials Characterization. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012. https://doi.org/10.1007/978-3-642-20620-7_14
Hauser H, Jiles DC, Melikhov Y, et al. An approach to modeling the dependence of magnetization on magnetic field in the high field regime. J Magn Magn Mater 2006;300(2):273–83. https://doi.org/10.1016/j.jmmm.2005.05.017
Kaur J, Ledward DA, Park RWA, et al. Factors affecting the heat resistance of Escherichia coli O157 : H7. Lett Appl Microbiol 1998;26(4):325–30. https://doi.org/10.1046/j.1472-765X.1998.00339.x PMid: 9633099
Abrinaei F, Kimiagar S, Zolghadr S. Hydrothermal synthesis of hematite-GO nanocomposites at different GO contents and potential application in nonlinear optics. Opt Mater 2019;96:109285. https://doi.org/10.1016/j.optmat.2019.109285
Al-Ruqeishi MS, Mohiuddin T, Al-Moqbali M, et al. Graphene oxide synthesis: Optimizing the Hummers and Marcano methods. Nanoscience and Nanotechnology Letters 2020;12(1):88–95. https://doi.org/10.1166/nnl.2020.3074
Santoro G, Domingo C. Espectroscopía Raman de nanotubos de carbono. Opt Pura Apl 2007;40(2):175–86.
Dresselhaus M, Dresselhaus G, Saito R, et al. Raman spectroscopy of carbon nanotubes. Phys Rep 2005;409(2) https://doi.org/10.1016/j.physrep.2004.10.006
Markovi? Z, Jovanovi? S, Kleut D, et al. Comparative study on modification of single wall carbon nanotubes by sodium dodecylbenzene sulfonate and melamine sulfonate superplasticiser. Appl Surf Sci 2009;255(12):6359–66. https://doi.org/10.1016/j.apsusc.2009.02.016
Bokobza L, Zhang J. Raman spectroscopic characterization of multiwall carbon nanotubes and of composites. Express Polym Lett 2012;6(7):601–8. https://doi.org/10.3144/expresspolymlett.2012.63 PMid: 25435600
Cançado LG, Jorio A, Ferreira EHM, et al. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett 2011;11(8):3190–6. https://doi.org/10.1021/nl201432g PMid: 21696186
Hafiz SM, Ritikos R, Whitcher TJ, et al. A practical carbon dioxide gas sensor using room-temperature hydrogen plasma reduced graphene oxide. Sens Actuators B Chem 2014;193:692–700. https://doi.org/10.1016/j.snb.2013.12.017
Ramirez S, Chan K, Hernandez R, et al. Thermal and magnetic properties of nanostructured densified ferrimagnetic composites with graphene - graphite fillers. Mater Des 2017;118:75–80. https://doi.org/10.1016/j.matdes.2017.01.018
Satheesh M, Paloly AR, Krishna Sagar CK, et al. Improved coercivity of solvothermally grown hematite (??Fe2O3) and hematite/graphene oxide nanocomposites (??Fe2O3/GO) at low temperature. physica status solidi (a) 2018;215(2):1700705. https://doi.org/10.1002/pssa.201700705
Romero MP, Gobo NRS, de Oliveira KT, et al. Photophysical properties and photodynamic activity of a novel menthol–zinc phthalocyanine conjugate incorporated in micelles. J Photochem Photobiol A Chem 2013;253:22–9. https://doi.org/10.1016/j.jphotochem.2012.12.009
Wang Y, Song X, Shao S, et al. An efficient, soluble, and recyclable multiwalled carbon nanotubes-supported TEMPO for oxidation of alcohols. RSC Adv 2012;2(20):7693. https://doi.org/10.1039/c2ra21206d
Bera M, Chandravati P, Gupta P, et al. Facile one-pot synthesis of graphene oxide by sonication assisted mechanochemical approach and its surface chemistry. J Nanosci Nanotechnol 2018;18(2):902–12. https://doi.org/10.1166/jnn.2018.14306 PMid: 29448514
Chen P, Marshall AS, Chi S, et al. Luminescent quadrupolar borazine oligomers: Synthesis, photophysics, and two?photon absorption properties. Chemistry – A European Journal 2015;21(50):18237–47. https://doi.org/10.1002/chem.201502268 PMid: 26514664
Meier H, Gerold J, Kolshorn H, et al. Extension of conjugation leading to bathochromic or hypsochromic effects in OPV series. Chemistry 2004;10(2):360–70. https://doi.org/10.1002/chem.200305447 PMid: 14735504
Wu W, Shen J, Gai Z, et al. Multi-functional core-shell hybrid nanogels for pH-dependent magnetic manipulation, fluorescent pH-sensing, and drug delivery. Biomaterials 2011;32(36):9876–87. https://doi.org/10.1016/j.biomaterials.2011.08.082 PMid: 21944827
Wu W, Shen J, Banerjee P, et al. Core–shell hybrid nanogels for integration of optical temperature-sensing, targeted tumor cell imaging, and combined chemo-photothermal treatment. Biomaterials 2010;31(29):7555–66. https://doi.org/10.1016/j.biomaterials.2010.06.030 PMid: 2064348
Sánchez-Iglesias A, Grzelczak M, Rodríguez-González B, et al. Synthesis of multifunctional composite microgels via in situ Ni growth on pNIPAM-coated Au nanoparticles. ACS Nano 2009;3(10):3184–90. https://doi.org/10.1021/nn9006169 PMid: 19769339
Begines B, Ortiz T, Pérez-Aranda M, et al. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020;10(7):1403. https://doi.org/10.3390/nano10071403 PMid: 32707641
Romero MP, Marangoni VS, de Faria CG, et al. Graphene oxide mediated broad-spectrum antibacterial based on bimodal action of photodynamic and photothermal effects. Front Microbiol 2020;10:2995. https://doi.org/10.3389/fmicb.2019.02995 PMid: 32010081
Dong X, Sun Z, Wang X, et al. An innovative MWCNTs/DOX/TC nanosystem for chemo-photothermal combination therapy of cancer. Nanomedicine 2017;13(7):2271–80. https://doi.org/10.1016/j.nano.2017.07.002
Lim JH, Kim DE, Kim E-J, et al. Functional graphene oxide-based nanosheets for photothermal therapy. Macromol Res 2018;26(6):557–65. https://doi.org/10.1007/s13233-018-6067-3 PMid: 32062387
Tager AA, Safronov AP, Sharina SV, et al. Thermodynamic study of poly(N-vinyl caprolactam) hydration at temperatures close to lower critical solution temperature. Colloid Polym Sci 1993;271(9):868–72. https://doi.org/10.1007/BF00652769
Kou Y, Wang S, Luo J, et al. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J Chem Thermodyn 2019;128:259–74. https://doi.org/10.1016/j.jct.2018.08.031
Grössinger R. Correlation between the inhomogeneity and the magnetic anisotropy in polycrystalline ferromagnetic materials. J Magn Magn Mater 1982;28(1–2):137–42. https://doi.org/10.1016/0304-8853(82)90037-3
Batlle X, García del Muro M, Tejada J, et al. Magnetic study of M-type doped barium ferrite nanocrystalline powders. J Appl Phys 1993;74(5):3333–40. https://doi.org/10.1063/1.354558
Kodama RH. Magnetic nanoparticles. J Magn Magn Mater 1999;200(1–3):359–72. https://doi.org/10.1016/S0304-8853(99)00347-9
Zhang D, Klabunde K, Sorensen C, et al. Magnetization temperature dependence in iron nanoparticles. Phys Rev B Condens Matter Mater Phys 1998;58(21):14167–70. https://doi.org/10.1103/PHYSREVB.58.14167
Xiao G, Chien CL. Temperature dependence of spontaneous magnetization of ultrafine Fe particles in Fe-SiO2 granular solids. J Appl Phys 1987;61(8):3308–10. https://doi.org/10.1063/1.338891
Cao XT, Showkat AM, Kang I, et al. ? -cyclodextrin multi-conjugated magnetic graphene oxide as a nano-adsorbent for methylene blue removal. J Nanosci Nanotechnol 2016;16(2):1521–5. https://doi.org/10.1166/jnn.2016.11987 PMid: 27433613
Amiri A, Baghayeri M, Sedighi M. Magnetic solid-phase extraction of polycyclic aromatic hydrocarbons using a graphene oxide/Fe3O4@polystyrene nanocomposite. Microchimica Acta 2018;185(8):1–9. https://doi.org/10.1007/s00604-018-2928-x
Mahdavi M, Ahmad MB, Haron MJ, et al. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013;18(7):7533–48. https://doi.org/10.3390/molecules18077533 PMid: 23807578
Donadel K, Felisberto MDV, Fávere VT, et al. Synthesis and characterization of the iron oxide magnetic particles coated with chitosan biopolymer. Materials Science and Engineering: C 2008;28(4):509–14. https://doi.org/10.1016/j.msec.2007.06.004
Qu J, Liu G, Wang Y, et al. Preparation of Fe3O4–chitosan nanoparticles used for hyperthermia. Advanced Powder Technology 2010;21(4):461–7. https://doi.org/10.1016/j.apt.2010.01.008
Huang P, Lin J, Yang D, et al. Photosensitizer-loaded dendrimer-modified multi-walled carbon nanotubes for photodynamic therapy. J Control Release 2011;152(supp 1):1:e33-4. https://doi.org/10.1016/j.jconrel.2011.08.105 PMid: 22195908
Xiao D, Qi H, Teng Y, et al. Advances and challenges of fluorescent nanomaterials for synthesis and biomedical applications. Nanoscale Res Lett 2021;16(1):167. https://doi.org/10.1186/s11671-021-03613-z PMid: 34837561
Ren Y, Liu H, Liu X, et al. Photoresponsive materials for antibacterial applications. Cell Rep Phys Sci 2020;1(11):100245. https://doi.org/10.1016/J.XCRP.2020.100245
Huo J, Jia Q, Huang H, et al. Emerging photothermal-derived multimodal synergistic therapy in combating bacterial infections. Chem Soc Rev 2021;50(15):8762–89. https://doi.org/10.1039/D1CS00074H PMid: 34159993
Liu Z, Zhao X, Yu B, et al. Rough carbon–iron oxide nanohybrids for near-infrared-II light-responsive synergistic antibacterial therapy. ACS Nano 2021;15(4):7482–90. https://doi.org/10.1021/acsnano.1c00894 PMid: 3385619
Mei L, Shi Y, Cao F, et al. PEGylated phthalocyanine-functionalized graphene oxide with ultrahigh-efficient photothermal performance for triple-mode antibacterial therapy. ACS Biomater Sci Eng 2021;7(6):2638–48. https://doi.org/10.1021/acsbiomaterials.1c00178 PMid: 33938721
Xu JW, Yao K, Xu ZK. Nanomaterials with a photothermal effect for antibacterial activities: An overview. Nanoscale 2019;11(18):8680–91. https://doi.org/10.1039/C9NR01833F PMid: 31012895
Hand JW, Haar G ter. Heating techniques in hyperthermia. Br J Radiol 1981;54(642):443–66. https://doi.org/10.1259/0007-1285-54-642-443 PMid:7016236
Hergt R, Dutz S, Müller R, Zeisberger M. Magnetic particle hyperthermia: nanoparticle magnetism and materials development for cancer therapy. Journal of Physics: Condensed Matter 2006;18(38):S2919–34. https://doi.org/10.1088/0953-8984/18/38/S26
Zhang L, Hou L, Zhang S, Kou X, Li R, Wang S. Mechanism of S. aureus ATCC 25923 in response to heat stress under different water activity and heating rates. Food Control 2020;108:106837. https://doi.org/10.1016/j.foodcont.2019.106837
Lagos KJ, Buzzá HH, Bagnato VS, Romero MP. Carbon-Based Materials in Photodynamic and Photothermal Therapies Applied to Tumor Destruction. Int J Mol Sci 2021;23(1):22. https://doi.org/10.3390/ijms23010022 PMid: 35008458
Mroz P, Yaroslavsky A, Kharkwal GB, et al. Cell death pathways in photodynamic therapy of cancer. Cancers 2011;3(2):2516–39. https://doi.org/10.3390/cancers3022516 PMid:23914299
Marangon I, Ménard-Moyon C, Silva AKA, et al. Synergic mechanisms of photothermal and photodynamic therapies mediated by photosensitizer/carbon nanotube complexes. Carbon 2016;97:110–23. https://doi.org/10.1016/j.carbon.2015.08.023
Naserzadeh P, Ansari Esfeh F, Kaviani M, et al. Single-walled carbon nanotube, multi-walled carbon nanotube and Fe2O3 nanoparticles induced mitochondria mediated apoptosis in melanoma cells. Cutan Ocul Toxicol 2018;37(2):157–66. https://doi.org/10.1080/15569527.2017.1363227 PMid: 28768445
Dias LD, Buzzá HH, Stringasci MD, et al. Recent advances in combined photothermal and photodynamic therapies against cancer using carbon nanomaterial platforms for in vivo studies. Photochem 2021;1(3):434–47. https://doi.org/10.3390/PHOTOCHEM1030026
Liu Z, Davis C, Cai W, et al. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proceedings of the National Academy of Sciences 2008;105(5):1410–5. https://doi.org/10.1073/pnas.0707654105 PMid: 18230737

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Copyright (c) 2025 Electronic Journal of Biotechnology